XENON, XE-133- xenon gas
Xenon, Xe-133 by
Drug Labeling and Warnings
Xenon, Xe-133 by is a Prescription medication manufactured, distributed, or labeled by Curium US LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Xenon Xe 133 Gas is for diagnostic inhalation use only. It is supplied in vials containing either 370 or 740 megabecquerels (10 or 20 millicuries) of Xenon Xe 133 Gas in 2 milliliters of carrier xenon and atmospheric air.
Xenon Xe 133 Gas is chemically and physiologically similar to elemental xenon, a non-radioactive gas which is physiologically inert except for anesthetic properties at high doses.
Xenon Xe 133 is produced by fission of Uranium U 235. At the time of calibration, it contains no more than 0.3% Xenon Xe 133m, no more than 1.5% Xenon Xe 131m, no more than 0.06% Krypton Kr 85 and no more than 0.01% Iodine I 131, with no less than 99.9% total radioactivity as radioxenon. Table 1 shows the effect of time on radionuclidic composition.
Table 1. Radionuclidic Composition
- *
Calibration Date
- † Expiration Date
Percent of Total Radioactivity
Days
% Xe-133
% Xe-133m
% Xe-131m
% Kr-85
% I-131
-5
>98.3
<0.6
<1.0
<0.03
<0.01
0*
>98.1
<0.3
<1.5
<0.06
<0.01
7
>97.2
<0.08
<2.5
<0.15
<0.02
14†
>95.7
<0.02
<4.1
<0.37
<0.02
- *
-
PHYSICAL CHARACTERISTICS
Xenon Xe 133 decays by beta and gamma emissions with a physical half-life of 5.245 days.1 Photons that are useful for detection and imaging studies as well as the principal beta emission are listed in Table 2.
Table 2. Principal Radiation Emission Data
Radiation
Mean % Per
Disintegration
Energy
(keV)
Beta-2
99.3
100.6 Avg.
Gamma-2
36.5
81.0
K alpha x-rays
38.9
30.8 Avg.
K beta x-rays
9.1
35.0 Avg.
- 1
Kocher, David C., "Radioactive Decay Data Tables," DOE/TIC-11026, 138 (1981).
- 1
-
EXTERNAL RADIATION
The specific gamma ray constant for Xenon Xe 133 is 0.51 R/hr-mCi at 1 cm. The first half-value thickness is 0.0035 cm of Pb.
A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 3. For example, the use of 0.2 cm of Pb will decrease the external radiation exposure by a factor of about 1000.
Table 3. Radiation Attenuation by Lead Shielding
Shield
Thickness (Pb), cm
Coefficient
of Attenuation
0.0035
0.5
0.037
10-1
0.12
10-2
0.20
10-3
0.29
10-4
To correct for physical decay of this radionuclide, the fractions that remain at selected time intervals after the date of calibration are shown in Table 4.
Table 4. Physical Decay Chart; Xenon Xe 133, Half-life 5.245 Days
- *
Calibration Date
Days
Fraction
Remaining
Days
Fraction
Remaining
0*
1.000
8
0.347
1
0.876
9
0.304
2
0.768
10
0.267
3
0.673
11
0.234
4
0.589
12
0.205
5
0.516
13
0.179
6
0.453
14
0.157
7
0.397
- *
-
CLINICAL PHARMACOLOGY
Xenon Xe 133 is a readily diffusible gas which is neither utilized nor produced by the body. It passes through cell membranes, freely exchanges between blood and tissue, and tends to concentrate more in body fat than in blood, plasma, water or protein solutions. In the concentrations recommended for diagnostic studies, it is physiologically inactive. Inhaled Xenon Xe 133 Gas will enter the alveolar wall and the pulmonary venous circulation via capillaries. Most of the Xenon Xe 133 Gas that enters the circulation from a single breath is returned to the lungs and exhaled after a single pass through the peripheral circulation.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Xenon Xe 133 Gas delivery systems, i.e., respirators or spirometers, and associated tubing assemblies must be leakproof to avoid loss of radioactivity into the laboratory environs not specifically protected by exhaust systems.
Xenon Xe 133 Gas adheres to some plastics and rubber and should not be allowed to stand in tubing or respirator containers. Loss of radioactivity due to such adherence may render the study nondiagnostic.
-
PRECAUTIONS
General
Xenon Xe 133 Gas as well as other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Also, care should be taken to minimize radiation exposure to the patients consistent with proper patient management.
Exhaled Xenon Xe 133 Gas should be controlled in a manner that is in compliance with the appropriate regulations of the government agency authorized to license the use of radionuclides.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential, mutagenic potential or whether this drug affects fertility in males or females.
Pregnancy Category C
Animal reproduction studies have not been conducted with Xenon Xe 133 Gas. It is also not known whether Xenon Xe 133 Gas can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Xenon Xe 133 Gas should be given to a pregnant woman only if clearly needed.
Ideally, all examinations that use radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability should be performed during the first few (approximately 10) days following the onset of menses.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Xenon Xe 133 Gas is administered by inhalation from a closed respirator system or spirometer. The final patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
The recommended activity range employed for inhalation by the average patient (70 kg) is:
Pulmonary function including imaging: 74 to 1110 megabecquerels (2 to 30 millicuries)
Cerebral blood flow: 370 to 1110 megabecquerels (10 to 30 millicuries)
This may be administered as a bolus into the tubing near the patient's mouthpiece or mask after the completion of a tidal exhalation, or by rebreathing for a period of approximately 5 minutes of the Xenon Xe 133 gas in equilibrium with the air contained in the closed system at concentrations of the radionuclide that may vary from 37 to 222 megabecquerels (1.0 to 6.0 millicuries) per liter.
-
RADIATION DOSIMETRY
The estimated absorbed radiation doses to an average patient (70 kg) for inhalation studies from a maximum dose of Xenon Xe 133 gas in 5, 7.5 and 10 liters are shown in Table 5. The values are the maximum absorbed dose that could be anticipated under the given conditions.
Table 5. Radiation Dose Estimates of Xenon Xe 1332: Absorbed Dose per 1110 megabecquerels (30 millicuries) of Xenon Xe 133 Gas Administered by Inhalation
Tissue
Spirometer Volume (liters)
5
7.5
10
Absorbed Radiation Doses
mGy
Rad
mGy
Rad
mGy
Rad
Lung
3.3
0.33
2.46
0.246
1.95
0.195
Red Marrow
0.45
0.045
0.36
0.036
0.27
0.027
Ovaries
0.39
0.039
0.30
0.030
0.24
0.024
Testes
0.36
0.036
0.27
0.027
0.21
0.021
Total Body
0.42
0.042
0.33
0.033
0.27
0.027
- 2
Atkins, Harold L., et al., Estimates of Radiation Absorbed Doses from Radioxenons in Lung Imaging. Task Group of the Medical Internal Radiation Dose Committee, Society of Nuclear Medicine, J. Nucl. Med., 21:459-465,1980.
- 2
-
DIRECTIONS FOR DISPENSING
Transfer the appropriate Xenon Xe 133 Gas dose from the Xenon Xe 133 Gas unit dose vial(s) to a breathing device or spirometer utilizing the Xenotron™ I Xenon Gas Dispenser. Follow the directions for use that are provided with the Xenotron™ I Xenon Gas Dispenser.
Xenon Xe 133 Gas should not be used after 14 days from the date of calibration stated on the label.
-
ACTIVITY MEASUREMENTS
Calibrate a suitable commercial ionization chamber dose calibrator according to the manufacturer's instructions for that particular instrument. An instrument that gives direct radioactivity readouts is recommended.
Use a National Institute of Standards and Technology (NIST) Xenon Xe 133 standard for the initial calibration. Also establish a secondary standard, such as Americium Am 241, at that time for subsequent routine use. Other suitable radionuclides may also be used. Determine the effective readout of the secondary standard compared to the Xenon Xe 133 standard over the range of activities expected for routine measurements. Determine the radioactivities of the dose for administration as follows:
- Check the dose calibrator for proper response with the secondary standard.
- Insert the Xenon Xe 133 Gas unit dose vial in the dose calibrator and measure the apparent radioactivity of the Xenon Xe 133.
- Correct for decay as necessary.
The radioactivity determined by this method is within 25% of the true value. This degree of accuracy includes variations attributed to small differences in geometry and radiation attenuation between the NIST standard ampule and the Xenon Xe 133 Gas unit dose vial.
- HOW SUPPLIED
-
STORAGE
Xenon Xe 133 Gas should be stored at controlled room temperature 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
The U.S. Nuclear Regulatory Commission has approved distribution of this radiopharmaceutical to persons licensed to use byproduct material listed in Sections 35.200 and to persons who hold an equivalent license issued by an Agreement State.
Curium and the Curium logo are trademarks of a Curium company.
©2018 Curium US LLC. All Rights Reserved.
Curium US LLC
Maryland Heights, MO 63043 USAA097I0
R12/2018
CURIUM™
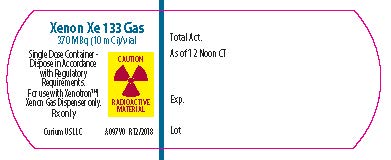
- PRINCIPAL DISPLAY PANEL - 10 mCi
- PRINCIPAL DISPLAY PANEL - 20 mCi
-
INGREDIENTS AND APPEARANCE
XENON, XE-133
xenon gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69945-097 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XENON XE-133 (UNII: X3P9A5HNYF) (XENON XE-133 - UNII:X3P9A5HNYF) XENON XE-133 5 mCi in 1 mL Inactive Ingredients Ingredient Name Strength AIR (UNII: K21NZZ5Y0B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69945-097-11 1 in 1 CONTAINER 03/30/2016 1 1 in 1 CAN 1 2 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 69945-097-13 3 in 1 CONTAINER 03/30/2016 2 1 in 1 CAN 2 2 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 69945-097-15 5 in 1 CONTAINER 03/30/2016 3 1 in 1 CAN 3 2 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018327 03/30/2016 XENON, XE-133
xenon gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69945-098 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XENON XE-133 (UNII: X3P9A5HNYF) (XENON XE-133 - UNII:X3P9A5HNYF) XENON XE-133 10 mCi in 1 mL Inactive Ingredients Ingredient Name Strength AIR (UNII: K21NZZ5Y0B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69945-098-21 1 in 1 CONTAINER 03/30/2016 1 1 in 1 CAN 1 2 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 69945-098-23 3 in 1 CONTAINER 03/30/2016 2 1 in 1 CAN 2 2 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 69945-098-25 5 in 1 CONTAINER 03/30/2016 3 1 in 1 CAN 3 2 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018327 03/30/2016 Labeler - Curium US LLC (079875617)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.