CERAVE ITCH RELIEF MOISTURIZING- pramoxine hydrochloride lotion
CeraVe Itch Relief Moisturizing by
Drug Labeling and Warnings
CeraVe Itch Relief Moisturizing by is a Otc medication manufactured, distributed, or labeled by VALEANT PHARMACEUTICALS NORTH AMERICA LLC, Accupac, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive ingredients
water, isopropyl myristate, cetyl alcohol, stearic acid, cetearyl alcohol, glyceryl stearate, PEG-100 stearate, glycerin, dimethicone, niacinamide, ceramide 3, ceramide 6-II, ceramide 1, hyaluronic acid, phenoxyethanol, xanthan gum, behentrimoium methosulfate, polyglyceryl-3 diisostearate, sodium hydroxide, alcohol denat., allantoin, ethylhexylglycerin, sodium lauroyl lactylate, arginine PCA,
disodium EDTA, potassium phosphate, dipotassium phosphate, tasmannia lanceolata fruit extract, zinc citrate, sodium PCA, phytosphingosine, cholesterol, carbomer, tocopheryl acetate
- Questions
-
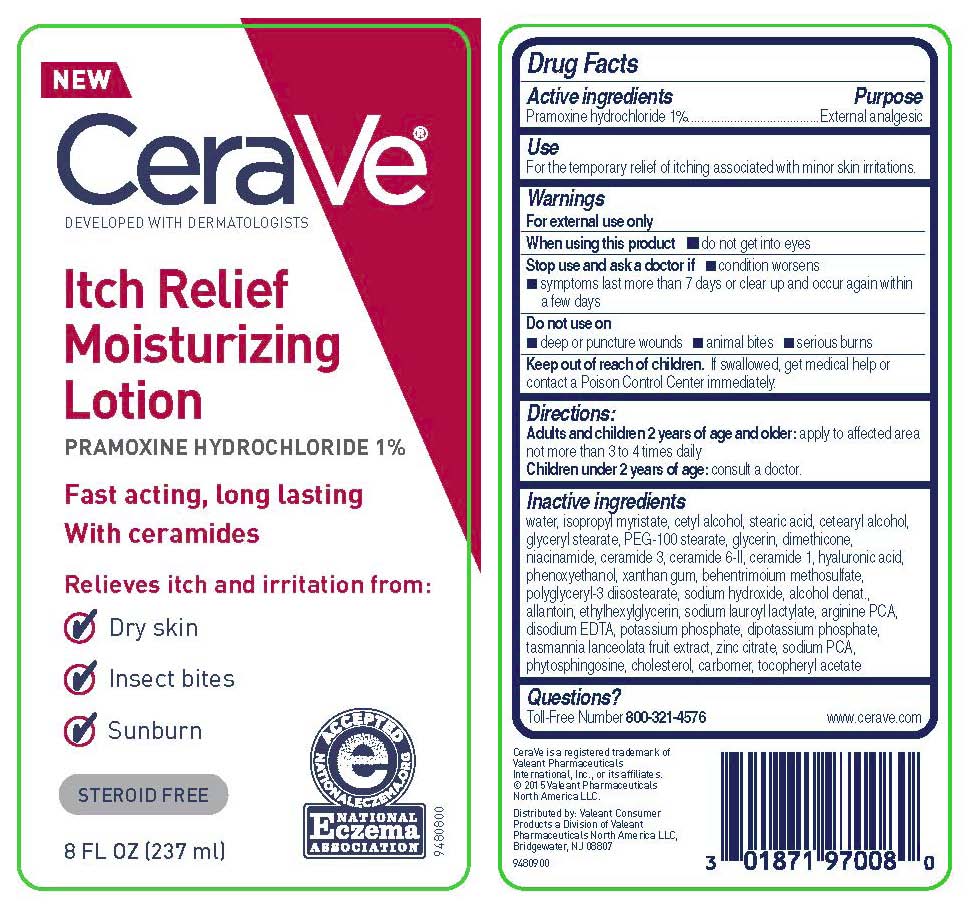
Principal Display Panel
NEW
CeraVe®
DEVELOPED WITH DERMATOLOGISTS
Itch Relief
Moisturizing
Lotion
PRAMOXINE HYDROCHLORIDE 1%
Fast acting, long lasting
With ceramides
Relieves itch and irritation from:
[check mark icon] Dry skin
[check mark icon] Insect bites
[check mark icon] Sunburn
STEROID FREE
[icon - National Eczema Association Seal]
8 FL OZ (237 mL)
-
INGREDIENTS AND APPEARANCE
CERAVE ITCH RELIEF MOISTURIZING
pramoxine hydrochloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0187-1970 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CETYL ALCOHOL (UNII: 936JST6JCN) STEARIC ACID (UNII: 4ELV7Z65AP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) NIACINAMIDE (UNII: 25X51I8RD4) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) HYALURONIC ACID (UNII: S270N0TRQY) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALCOHOL (UNII: 3K9958V90M) ALLANTOIN (UNII: 344S277G0Z) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) ARGININE PYROGLUTAMATE (UNII: 808T94CEU6) EDETATE DISODIUM (UNII: 7FLD91C86K) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) TASMANNIA LANCEOLATA FRUIT (UNII: PNT2HDL13Q) ZINC CITRATE (UNII: K72I3DEX9B) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-1970-08 237 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2016 2 NDC: 0187-1970-99 15 in 1 TRAY 02/01/2016 2 29.6 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/18/2016 Labeler - VALEANT PHARMACEUTICALS NORTH AMERICA LLC (042230623) Establishment Name Address ID/FEI Business Operations Accupac, Inc. 071609663 MANUFACTURE(0187-1970)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.