Dextromethorphan HBr by Rugby Laboratories / LNK International, Inc. Rugby 44-732-Delisted
Dextromethorphan HBr by
Drug Labeling and Warnings
Dextromethorphan HBr by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEXTROMETHORPHAN HBR- dextromethorphan hbr capsule, liquid filled
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Rugby 44-732-Delisted
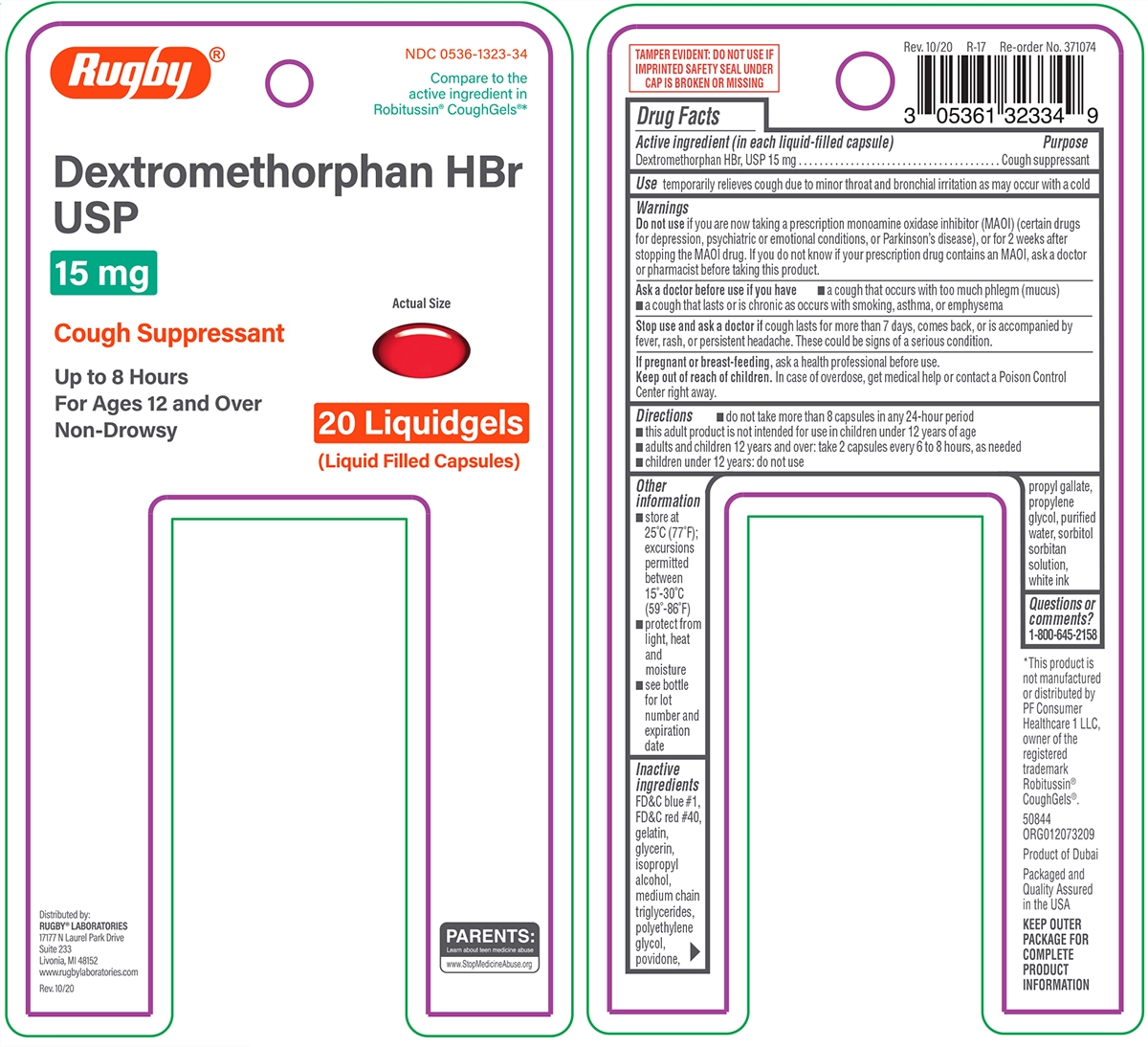
Use
temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that occurs with too much phlegm (mucus)
- a cough that lasts or is chronic as occurs with smoking, asthma, or emphysema
Directions
- do not take more than 8 capsules in any 24-hour period
- this adult product is not intended for use in children under 12 years of age
- adults and children 12 years and over: take 2 capsules every 6 to 8 hours, as needed
- children under 12 years: do not use
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from light, heat and moisture
- see bottle for lot number and expiration date
Inactive ingredients
FD&C blue #1, FD&C red #40, gelatin, glycerin, isopropyl alcohol, medium chain triglycerides, polyethylene glycol, povidone, propyl gallate, propylene glycol, purified water, sorbitol sorbitan solution, white ink
Principal display panel
Rugby®
NDC: 0536-1323-34
Compare to the
active ingredient in
Robitussin® CoughGels®*
Dextromethorphan HBr
USP
15 mg
Cough Suppressant
Up to 8 Hours
For Ages 12 and Over
Non-Drowsy
20 Liquidgels
(Liquid Filled Capsules)
Actual Size
Distributed by:
RUGBY® LABORATORIES
17177 N Laurel Park Drive
Suite 233
Livonia, MI 48152
www.rugbylaboratories.com
Rev. 10/20
PARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.org
*This product is
not manufactured
or distributed by
PF Consumer Healthcare 1 LLC,
owner of the
registered
trademark Robitussin®
CoughGels®.
50844 ORG012073209
Product of Dubai
Packaged and
Quality Assured
in the USA
KEEP OUTER
PACKAGE FOR
COMPLETE
PRODUCT
INFORMATION
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSING
Rev. 10/20 R-17 Re-Order No. 371074
Rugby 44-732
| DEXTROMETHORPHAN HBR
dextromethorphan hbr capsule, liquid filled |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(0536-1323) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(0536-1323) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.