BUDESONIDE spray, metered
Budesonide by
Drug Labeling and Warnings
Budesonide by is a Otc medication manufactured, distributed, or labeled by Topco Associates LLC, Apotex Inc., Legacy Pharmaceutical Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
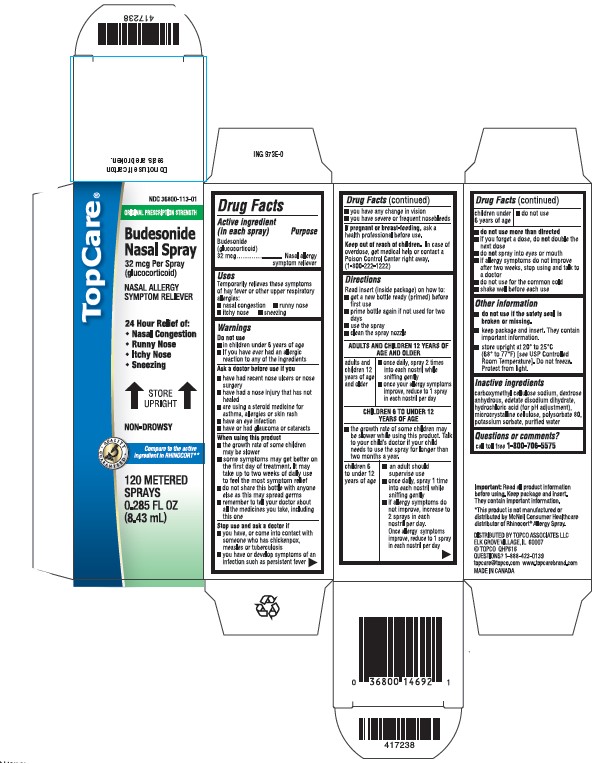
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
Do not use
- in children under 6 years of age
- if you have ever had an allergic reaction to any of the ingredients
Ask a doctor before use if you
- have had recent nose ulcers or nose surgery
- have had a nose injury that has not healed
- are using a steroid medicine for asthma, allergies or skin rash
- have an eye infection
- have or had glaucoma or cataracts
When using this product
- the growth rate of some children may be slower
- some symptoms may get better on the first day of treatment. It may take up to two weeks of daily use to feel the most symptom relief.
- do not share this bottle with anyone else as this may spread germs
- remember to tell your doctor about all the medicines you take, including this one
-
Directions
Read insert (inside package) on how to:
- get a new bottle ready (primed) before first use
- prime bottle again if not used for two days
- use the spray
- clean the spray nozzle
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
adults and children 12 years of age and older
- once daily, spray 2 times into each nostril while sniffing gently
- once your allergy symptoms improve, reduce to 1 spray in each nostril per day
CHILDREN 6 TO UNDER 12 YEARS OF AGE - the growth rate of some children may be slower while using this product. Talk to your child’s doctor if your child needs to use the spray for longer than two months a year
children 6 to under 12 years of age - an adult should supervise use
- once daily, spray 1 time into each nostril while sniffing gently
- if allergy symptoms do not improve, increase to 2 sprays in each nostril per day. Once allergy symptoms improve, reduce to 1 spray in each nostril per day
children under 6 years of age - do not use
- do not use more than directed
- if you forget a dose, do not double the next dose
- do not spray into eyes or mouth
- if allergy symptoms do not improve after two weeks, stop using and talk to a doctor
- do not use for the common cold
- shake well before each use
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel - Carton
CARTON LABEL - PRINCIPAL DISPLAY PANEL - 32 mcg per spray
TopCare NDC: 36800-113-01
Budesonide Nasal Spray
Allergy Spray
120 sprays
Relief of:
- Nasal Congestion
- Runny Nose
- Itchy Nose
- Sneezing
-
Principal Display Panel - Bottle
BOTTLE LABEL - PRINCIPAL DISPLAY PANEL - 32 mcg per spray
TopCare NDC: 36800-113-01
Budesonide Nasal Spray
Allergy Spray
120 sprays

-
INGREDIENTS AND APPEARANCE
BUDESONIDE
budesonide spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 36800-113 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Budesonide (UNII: Q3OKS62Q6X) (Budesonide - UNII:Q3OKS62Q6X) Budesonide 32 ug Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 36800-113-01 1 in 1 BOTTLE, SPRAY 08/31/2016 1 120 in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078949 08/31/2016 Labeler - Topco Associates LLC (006935977) Registrant - Apotex Inc. (209429182) Establishment Name Address ID/FEI Business Operations Apotex Inc. 255092496 analysis(36800-113) , manufacture(36800-113) Establishment Name Address ID/FEI Business Operations Legacy Pharmaceutical Packaging 143213275 pack(36800-113)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.