ACETAMINOPHEN tablet, film coated, extended release
Acetaminophen by
Drug Labeling and Warnings
Acetaminophen by is a Otc medication manufactured, distributed, or labeled by Shopko Stores Operating Co., LLC, Ranbaxy Pharmaceuticals Inc., Ohm Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
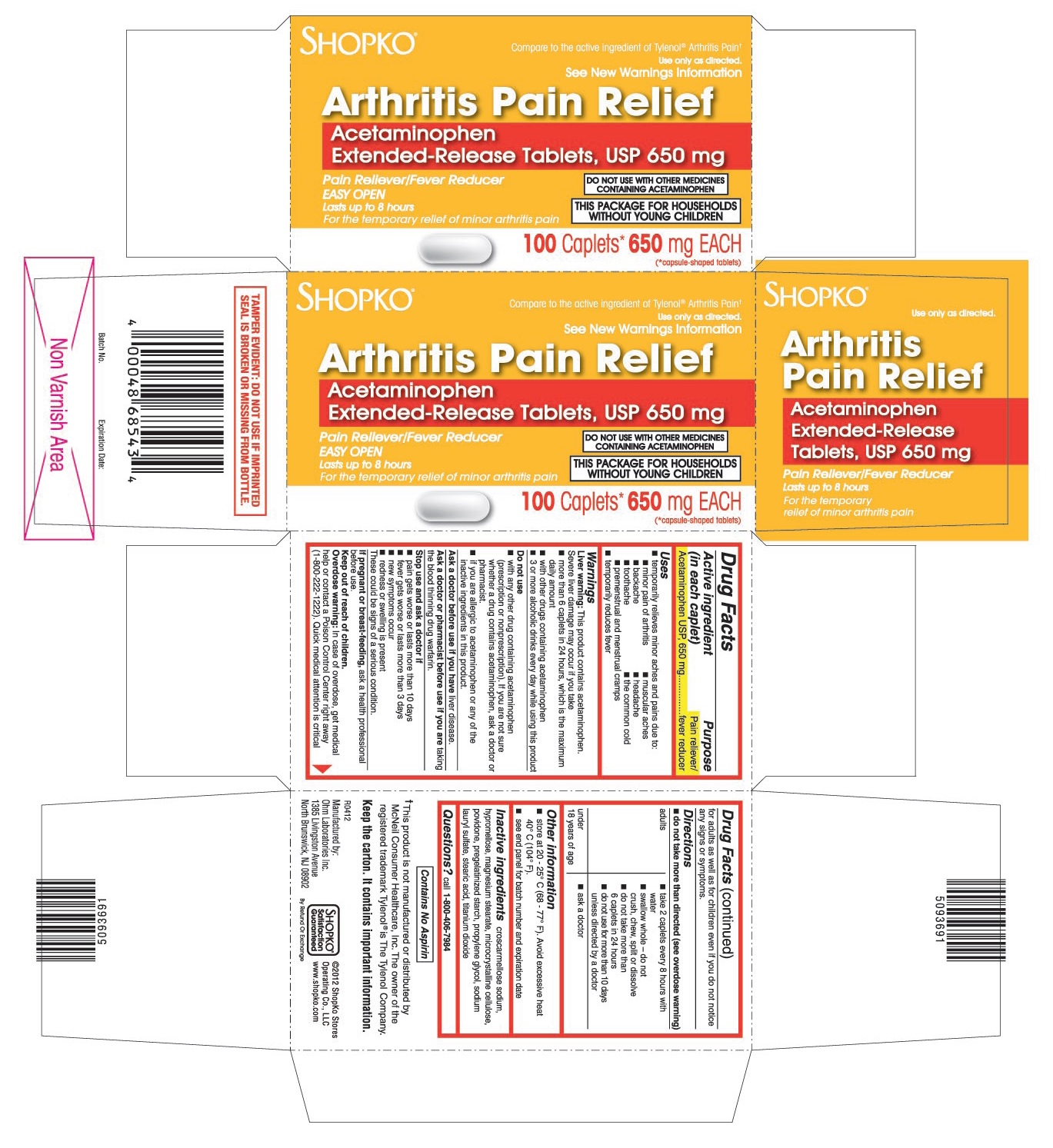
- ACTIVE INGREDIENT (IN EACH CAPLET)
- PURPOSE
- USES
-
WARNINGS
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
-
DIRECTIONS
-
do not take more than directed (see overdose warning)
adults ▪ take 2 caplets every 8 hours with water
▪ swallow whole - do not crush, chew, split or dissolve
▪ do not take more than 6 caplets in 24 hours
▪ do not use for more than 10 days unless directed by a doctorunder 18 years of age ask a doctor
-
do not take more than directed (see overdose warning)
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
PRINCIPAL DISPLAY PANEL
Compare to the active ingredient of Tylenol®Arthritis Pain†
Acetaminophen Extended-Release Tablets, USP 650 mg
DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN
For the temporary relief of minor arthritis pain
THIS PACKAGE FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tablet, film coated, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37012-333 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL (Capsule Shaped) Size 19mm Flavor Imprint Code cor116 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37012-333-01 100 in 1 BOTTLE 2 NDC: 37012-333-04 250 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076200 04/30/2002 Labeler - Shopko Stores Operating Co., LLC (023252638) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 manufacture(37012-333)
Trademark Results [Acetaminophen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.