LOVLUV VIVACITY BRIGHT CUSHION 21G- titanium dioxide, octinoxate, zinc oxide, octisalate liquid
LOVLUV Vivacity Bright Cushion 21g by
Drug Labeling and Warnings
LOVLUV Vivacity Bright Cushion 21g by is a Otc medication manufactured, distributed, or labeled by Cnt Dream. Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- OTC – ACTIVE INGREIDENT SECTION
-
INACTIVE INGREDIENT SECTION
Prunus Serrulata Flower Extract ,Cyclopentasiloxane,Butylene Glycol, Glycerin,
Cyclohexasiloxane,Sodium Hyaluronate,Caprylic/Capric Triglyceride,Cyclomethicone,
Trimethylsiloxysilicate,Arbutin,1,2-Hexanediol,Cetyl PEG/PPG-10/1 Dimethicone,
Iron Oxide Yellow(CI 77492),PEG-10 Dimethicone,Sodium Chloride,Sorbitan Sesquioleate,
Iron Oxide Red(CI 77491),Methicone,Triethoxycaprylylsilane,Camellia Japonica Seed Oil,
Xylitylglucoside,Anhydroxylitol,Xylitol,Fragrance,Iron Oxide Black (CI 77499),
Ethylhexylglycerin,Adenosine - OTC – ASK DOCTOR SECTION
- OTC – DO NO USE SECTION
- OTC – KEEP OUT OF REACH OF CHILDREN SECTION
-
WARNINGS SECTION

Warnings
*For external use only
*Do not use on damaged or broken skin
*When using this product keep out of eyes, Rinse with water to remove.
*Stop use and ask a doctor if rash or irritation develops and lasts.
*Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away - OTC – PURPOSE SECTION
-
INDICATIONS & USAGE SECTION

Uses
*helps prevent sunburn
*if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sunDirections:
Apply a proper amount evenly for the last step of skin care.
For sunscreen use:
*apply liberally 15 minutes before sun exposure
*reapply at least every 2 hours
*use a water resistant sunscreen if swimming or sweating
*Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
*limit time in the sun, especially from 10a.m-2p.m
*wear long-sleeved shirts, pants, hats, and sunglasses
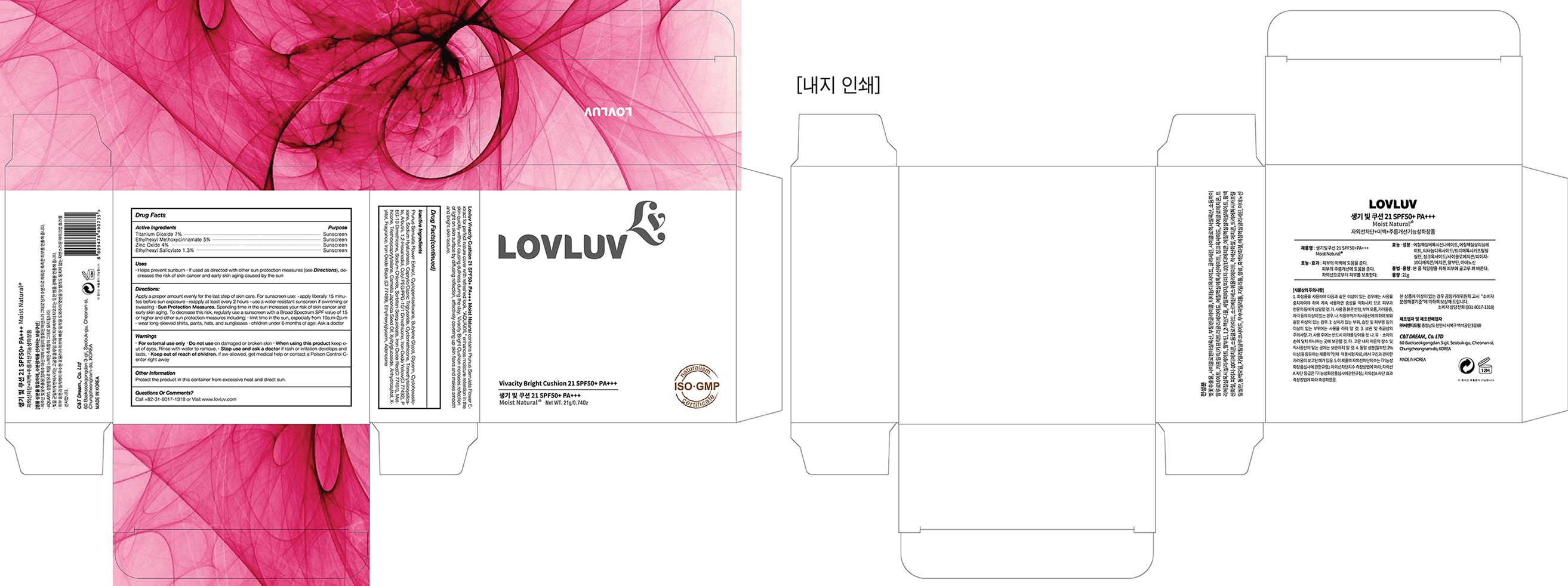
*children under 6 months of age: Ask a doctor - PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LOVLUV VIVACITY BRIGHT CUSHION 21G
titanium dioxide, octinoxate, zinc oxide, octisalate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71909-0735 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.47 g in 21 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.84 g in 21 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.05 g in 21 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.273 g in 21 g Inactive Ingredients Ingredient Name Strength PRUNUS SERRULATA FLOWER (UNII: 60I4615G0K) 5.7561 g in 21 g HYALURONATE SODIUM (UNII: YSE9PPT4TH) 1.05 g in 21 g Product Characteristics Color brown Score no score Shape ROUND Size 85mm Flavor TANGERINE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71909-0735-1 1 in 1 BOX 01/31/2018 12/31/2018 1 NDC: 71909-0735-0 21 g in 1 BOTTLE, PLASTIC; Type 1: Convenience Kit of Co-Package 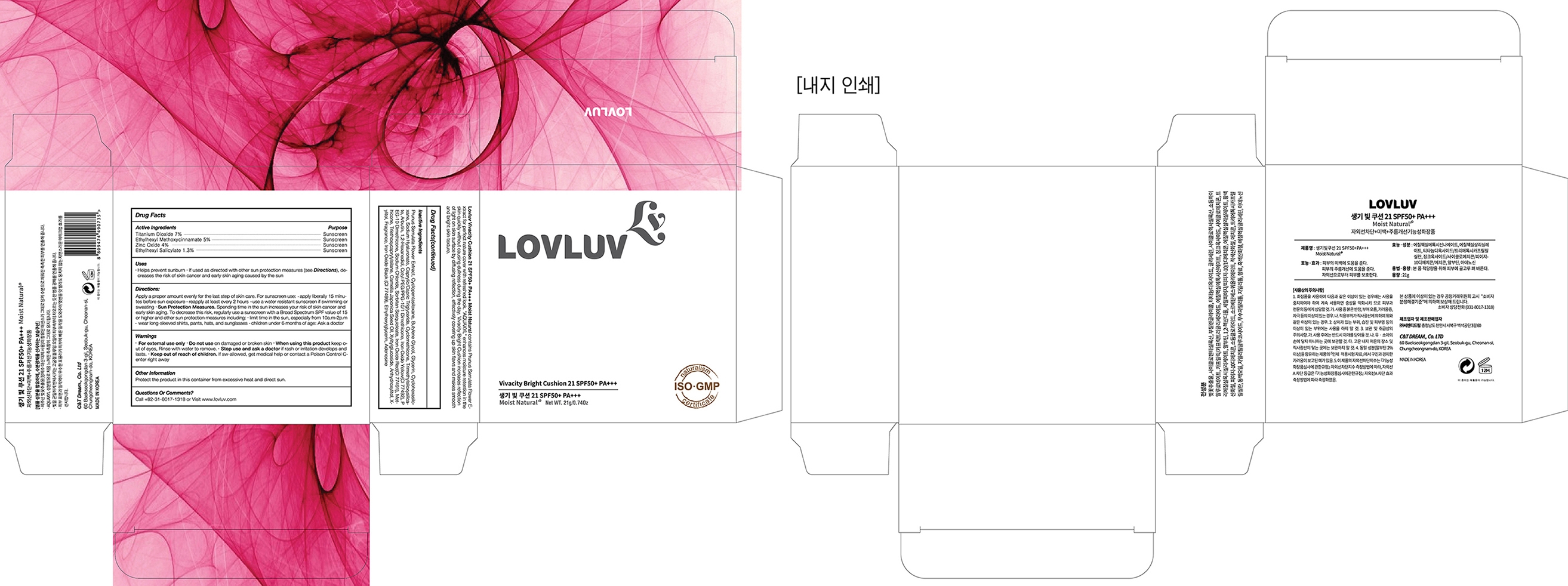
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/31/2018 Labeler - Cnt Dream. Co., Ltd (694699750) Registrant - Cnt Dream. Co., Ltd (694699750) Establishment Name Address ID/FEI Business Operations Cnt Dream. Co., Ltd 694699750 manufacture(71909-0735)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.