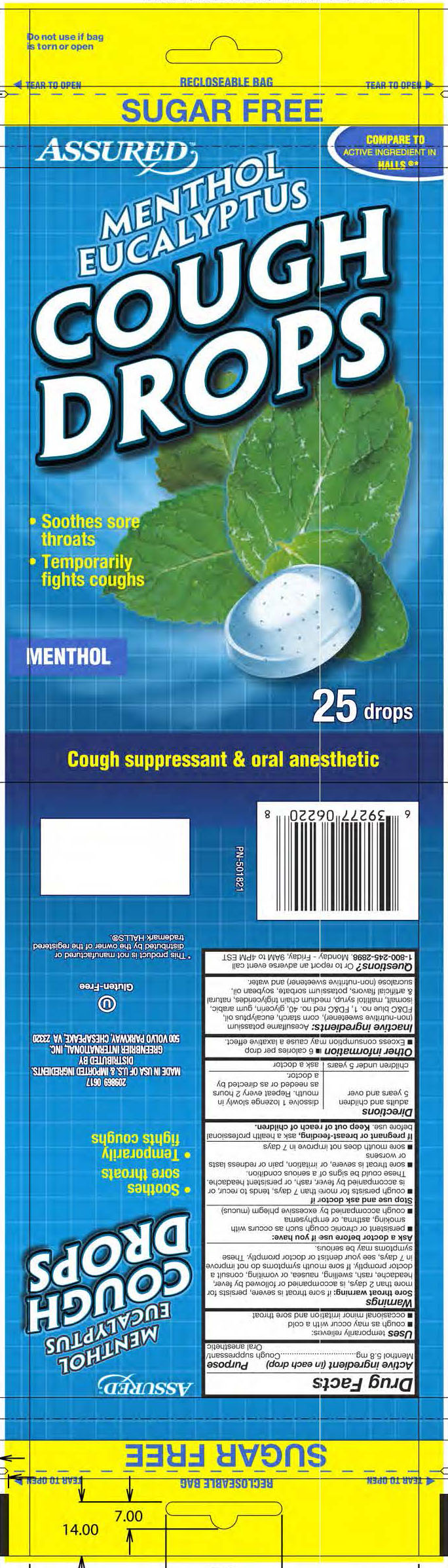
SUGAR FREE MENTHOL COUGH DROPS- menthol lozenge
sugar free menthol cough drops by
Drug Labeling and Warnings
sugar free menthol cough drops by is a Otc medication manufactured, distributed, or labeled by Greenbrier International Inc., Bestco Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, see your dentist or doctor promptly. These symptoms may be serious.
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients: acesulfame potassium (non-nutritive sweetener), corn starch, eucalyptus oil, FD&C blue no. 1, FD&C red no. 40, glycerin, gum arabic, isomalt, maltitol syrup, medium chain triglycerides, natural and artificial flavors, potassium sorbate, soybean oil, sucralose (non-nutritive sweetener), and water.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUGAR FREE MENTHOL COUGH DROPS
menthol lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 33992-1015 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.8 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color blue (light blue with blue flakes) Score no score Shape OVAL Size 17mm Flavor MENTHOL Imprint Code B Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33992-1015-1 25 in 1 BAG; Type 0: Not a Combination Product 12/07/2017 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/07/2017 Labeler - Greenbrier International Inc. (610322518) Registrant - Bestco Inc. (002149136) Establishment Name Address ID/FEI Business Operations Bestco Inc. 002149136 manufacture(33992-1015)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.