SKINUVA SCAR PLUS- zinc oxide cream
Skinuva Scar plus by
Drug Labeling and Warnings
Skinuva Scar plus by is a Otc medication manufactured, distributed, or labeled by MD Medical Designs, Inc., Dermazone LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water/Aqua, Dimethicone, Dimethiconol, Cyclopentasiloxane, Dimethicone Crosspolymer, Butylene Glycol, Glycerin, Diethylhexyl 2,6-Naphthalate, Dimethicone/PEG-10/15 Crosspolymer, Tetrahexyldecyl Ascorbate, Centella Asiatica Extract, Diphenylsiloxy Phenyl Trimethicone, Dimethicone/Phenyl Vinyl Dimethicone Crosspolymer, Phenoxyethanol, Ethylhexylglycerin, Sodium Chloride, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Sodium Hyaluronate, Aloe Barbadensis Leaf Juice, sh-Polypeptide-1, Tocopherol, sh-Polypeptide-6, sh-Polypeptide-5
- SPL UNCLASSIFIED SECTION
-
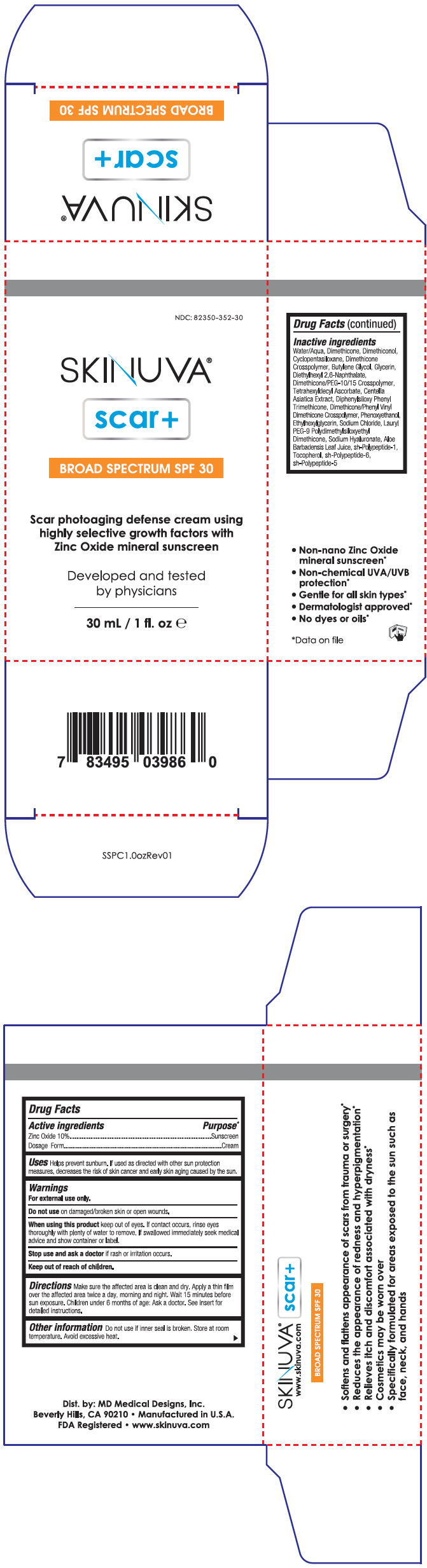
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
NDC: 82350-352-30
SKINUVA®
scar+BROAD SPECTRUM SPF 30
Scar photoaging defense cream using
highly selective growth factors with
Zinc Oxide mineral sunscreenDeveloped and tested
by physicians30 mL / 1 fl. oz e
-
INGREDIENTS AND APPEARANCE
SKINUVA SCAR PLUS
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82350-352 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.1 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIMETHICONE/PHENYL VINYL DIMETHICONE CROSSPOLYMER (UNII: ST6ZC4KVH2) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM CHLORIDE (UNII: 451W47IQ8X) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALOE VERA LEAF (UNII: ZY81Z83H0X) BASIC FIBROBLAST GROWTH FACTOR (HUMAN) (UNII: S3529G9M9V) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) INTERLEUKIN-10 (UNII: 9SC4O216V9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82350-352-15 1 in 1 CARTON 06/30/2022 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 82350-352-30 1 in 1 CARTON 06/30/2022 2 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M020 06/30/2022 Labeler - MD Medical Designs, Inc. (094586004) Establishment Name Address ID/FEI Business Operations Dermazone LLC 131639839 MANUFACTURE(82350-352) , PACK(82350-352)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.