BENZODENT- benzocaine cream
Benzodent by
Drug Labeling and Warnings
Benzodent by is a Otc medication manufactured, distributed, or labeled by Focus Consumer Healthcare, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
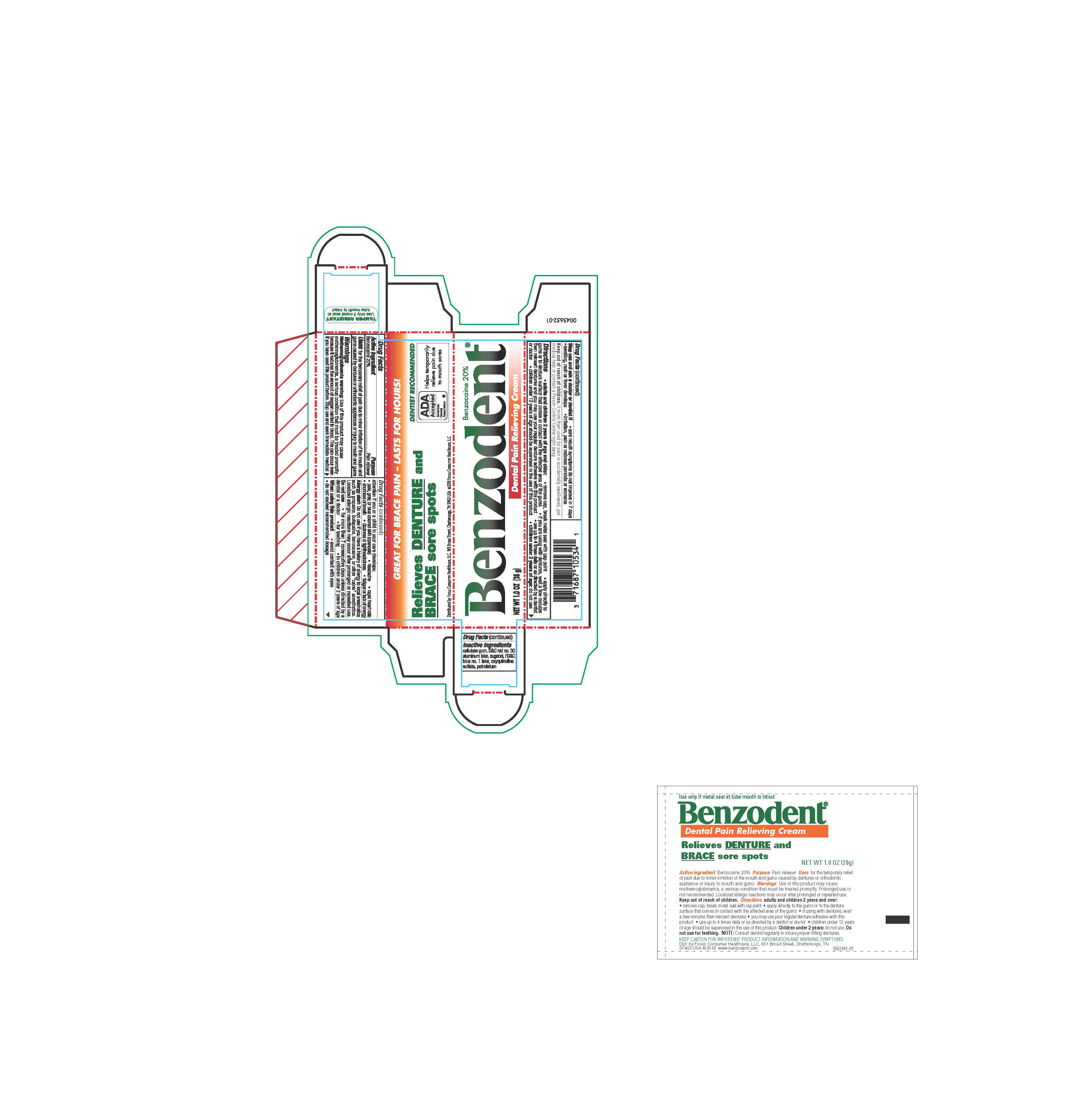
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Methemoglobinemia warning: Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried to the blood. This can occur even if you have used this product before. Stop use and seek immedicate medical attention if you or a child in your care develops:
- pale, gray or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Alllergy alert: Do not use if you have a history of allergy to local anetsthetics such as procaine, butacaine, benzocaine, or other "caine: anesthetics. Localized allergic reactions may occur after prolonged or repeated use.
Do not use
if you are allergic to aspirin or any other pain reliever/fever reducer
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if
- you have liver disease
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use
if you are taking a prescription drug for gout, diabetes or arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- feel faint
- redness or swelling is present
- new symptoms occur
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- ringing in the ears or loss of hearing occurs
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults and children12 years and over:
remove cap, break metal seal with cap point
apply directly to the gums or to the denture surface that comes in contact with the affected area of the gums
if you are using the dentures, wait a few minutes then reinsert dentures and you may use your regular denture adhesive with this product
- use up to 4 times daily or as directed by a dentist or doctor
- children under 12 years of age should be supervised in the use of this product
children under 12 years: consult a dentist or doctor
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENZODENT
benzocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71687-1053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.2 g in 1 g Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 30 (UNII: 2S42T2808B) EUGENOL (UNII: 3T8H1794QW) ALUMINUM OXIDE (UNII: LMI26O6933) OXYQUINOLINE SULFATE (UNII: 61VUG75Y3P) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71687-1053-1 1 in 1 CARTON 12/08/2017 1 7 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 71687-1053-2 1 in 1 CARTON 12/08/2017 2 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 12/08/2017 Labeler - Focus Consumer Healthcare, LLC (080743737)
Trademark Results [Benzodent]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BENZODENT 71651995 0595101 Live/Registered |
PETER STRONG & CO. INC. 1953-08-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.