FLUZONE TRIVALENT NORTHERN HEMISPHERE (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136/rv/2023 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension
FLUZONE TRIVALENT NORTHERN HEMISPHERE by
Drug Labeling and Warnings
FLUZONE TRIVALENT NORTHERN HEMISPHERE by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Fluzone® safely and effectively. See full prescribing information for Fluzone.
Fluzone (Influenza Vaccine)
injectable suspension, for intramuscular use
2025–2026 Formula
Initial U.S. Approval: 1980INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For intramuscular use (2)
Age Vaccination Status Dose Schedule "-" Indicates information is not applicable - * The schedule can be completed as two 0.25-mL doses ≥ 4 weeks apart, two 0.5-mL doses ≥ 4 weeks apart, or any combination of 2 doses (either 0.25 mL or 0.5 mL) administered ≥ 4 weeks apart.
- † To determine if 1 or 2 doses are required, refer to Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines
6 months through 35 months Not previously vaccinated with influenza vaccine or unknown vaccination history Two doses, either 0.25 mL or 0.5mL* Administer at least 4 weeks apart Previously vaccinated with influenza vaccine One or two doses†, either 0.25 mL or 0.5 mL* If two doses, administer at least 4 weeks apart 36 months through 8 years Not previously vaccinated with influenza vaccine or unknown vaccination history Two 0.5 mL doses Administer at least 4 weeks apart Previously vaccinated with influenza vaccine One or two 0.5 mL doses† If two doses, administer at least 4 weeks apart 9 years and older - One 0.5 mL dose - DOSAGE FORMS AND STRENGTHS
Fluzone is an injectable suspension.
For individuals 6 months through 35 months, a single dose is 0.25 mL or 0.5 mL.
For individuals 36 months and older, a single dose is 0.5 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
If Guillain-Barré syndrome (GBS) has occurred within 6 weeks of previous influenza vaccination, the decision to give Fluzone should be based on careful consideration of the potential benefits and risks. (5.1)
ADVERSE REACTIONS
- In children 6 months through 8 years of age, the most common injection-site adverse reactions were pain or tenderness (>50%) and redness (>25%); the most common solicited systemic adverse reactions were irritability and drowsiness (>25% of children 6 months through 35 months) and myalgia (>20% of children 3 years through 8 years). (6.1)
- In adults 18 through 64 years of age, the most common injection-site adverse reaction was pain (>50%); the most common solicited systemic adverse reactions were headache and myalgia (>30%). (6.1)
- In adults ≥65 years of age, the most common injection-site adverse reaction was pain (>20%); the most common solicited systemic adverse reactions were headache, myalgia, and malaise (>10%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc. at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
- Antibody responses to Fluzone are lower in persons ≥65 years of age than in younger adults. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
5.2 Preventing and Managing Allergic Reactions
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
5.5 Syncope
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy of Fluzone in Children 6 through 24 Months of Age
14.2 Efficacy of Fluzone in Adults
14.3 Immunogenicity of Fluzone in Children 6 Months through 8 Years of Age
14.4 Immunogenicity of a 0.5 mL Dose of Fluzone Quadrivalent in Children 6 Months through 35 Months of Age
14.5 Immunogenicity of Fluzone in Adults
14.6 Immunogenicity of Fluzone in Geriatric Adults
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use
2.1 Dose and Schedule
The dose and schedule for Fluzone are presented in Table 1.
Table 1: Dose and Schedule for Fluzone Age Vaccination Status Dose Schedule "-" Indicates information is not applicable - * The schedule can be completed as two 0.25-mL doses ≥ 4 weeks apart, two 0.5-mL doses ≥ 4 weeks apart, or any combination of 2 doses (either 0.25 mL or 0.5 mL) administered ≥ 4 weeks apart
- † To determine if 1 or 2 doses are required, refer to Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines
6 months through 35 months Not previously vaccinated with influenza vaccine or unknown vaccination history Two doses, either 0.25 mL or 0.5 mL* Administer at least 4 weeks apart Previously vaccinated with influenza vaccine One or two doses† , either 0.25 mL or 0.5 mL* If two doses, administer at least 4 weeks apart 36 months through 8 years Not previously vaccinated with influenza vaccine or unknown vaccination history Two 0.5 mL doses Administer at least 4 weeks apart Previously vaccinated with influenza vaccine One or two 0.5 mL doses† If two doses, administer at least 4 weeks apart 9 years and older - One 0.5 mL dose - 2.2 Administration
Fluzone is clear and slightly opalescent in color. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the vaccine should not be administered.
Before administering a dose of vaccine, shake the prefilled syringe or multi-dose vial. A maximum of ten doses can be withdrawn from the multi-dose vial.
Administer each dose intramuscularly.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer Fluzone to anyone with a history of severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)], including egg protein, or to a previous dose of any influenza vaccine.
-
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré syndrome (GBS) has occurred within 6 weeks following previous influenza vaccination, the decision to give Fluzone should be based on careful consideration of the potential benefits and risks.
The 1976 swine influenza vaccine was associated with an elevated risk of GBS. Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than 1 additional case per 1 million persons vaccinated. (1)
5.2 Preventing and Managing Allergic Reactions
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of Fluzone.
-
6 ADVERSE REACTIONS
In children 6 months through 8 years of age, the most common injection-site adverse reactions were pain or tenderness (>50%) and redness (>25%); the most common solicited systemic adverse reactions were irritability and drowsiness (>25% of children 6 months through 35 months) and myalgia (>20% of children 3 years through 8 years).
In adults 18 through 64 years of age, the most common injection-site adverse reaction was pain (>50%); the most common solicited systemic adverse reactions were headache and myalgia (>30%).
In adults ≥65 years of age, the most common injection-site adverse reaction was pain (>20%); the most common solicited systemic adverse reactions were headache, myalgia, and malaise (>10%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trial(s) of a vaccine cannot be directly compared to rates in the clinical trial(s) of another vaccine and may not reflect the rates observed in practice.
Children 6 Months through 8 Years of Age
Study 1 (NCT00391391) was a multi-center study conducted in the US. In this study, children 6 months through 35 months of age received two 0.25 mL doses of Fluzone, and children 3 years through 8 years of age received two 0.5 mL doses of Fluzone, irrespective of previous influenza vaccination history. The two doses (2006–2007 formulation) were administered 26 to 30 days apart. The safety analysis set included 97 children 6 months through 35 months of age and 163 children 3 years through 8 years of age. Table 2 and Table 3 summarize solicited injection site adverse reactions and systemic adverse reactions reported within 7 days post-vaccination via diary cards.
Table 2: Frequency of Solicited Injection Site and Systemic Adverse Reactions Within 7 Days After Vaccination with Fluzone, Children 6 Through 35 Months of Age (Study 1*) Dose 1 (N†=90–92)
PercentageDose 2 (N†=86–87)
PercentageAny Moderate‡ Severe§ Any Moderate‡ Severe§ - * (NCT00391391)
- † N is the number of vaccinated participants with available data for the adverse reactions listed
- ‡ Moderate - Injection-site tenderness: cries and protests when injection site is touched; Injection-site erythema, Injection-site swelling, Injection-site induration, and Injection-site ecchymosis: ≥2.5 cm to <5 cm; Fever: >101.3°F to ≤103.1°F; Vomiting: 2 to 5 episodes per 24 hours; Crying abnormal: 1 to 3 hours; Drowsiness: not interested in surroundings or did not wake up for a meal; Appetite lost: missed 1 or 2 feeds completely; Irritability: requiring increased attention
- § Severe - Injection-site tenderness: cries when injected limb is moved or the movement of the injected limb is reduced; Injection-site erythema, Injection-site swelling, Injection-site induration, and Injection-site ecchymosis: ≥5 cm; Fever: >103.1°F; Vomiting: ≥6 episodes per 24 hours or requiring parenteral hydration; Crying abnormal: >3 hours; Drowsiness: sleeping most of the time or difficulty to wake up; Appetite lost: refuses ≥3 feeds or refuses most feeds; Irritability: inconsolable
- ¶ Fever - The percentage of temperature measurements that were taken by rectal, axillary, or oral routes, or not recorded were 69.2%, 17.6%, 13.2%, and 0.0%, respectively, for Dose 1; and 69.0%, 13.8%, 16.1%, and 1.1%, respectively, for Dose 2
Injection-Site Tenderness 47.3 8.8 0.0 56.3 3.4 1.1 Injection-Site Erythema 29.3 0.0 0.0 32.2 1.1 0.0 Injection-Site Swelling 16.7 0.0 0.0 14.9 0.0 0.0 Injection-Site Induration 14.4 0.0 0.0 16.1 0.0 0.0 Injection-Site Ecchymosis 14.4 1.1 0.0 14.9 2.3 0.0 Fever¶ (≥100.4°F) 11.0 4.4 0.0 10.3 3.4 1.1 Vomiting 6.6 1.1 0.0 8.1 5.8 0.0 Crying Abnormal 31.9 11.0 0.0 18.6 7.0 2.3 Drowsiness 26.4 1.1 0.0 26.7 4.7 0.0 Appetite Lost 23.1 8.8 0.0 19.8 5.8 1.2 Irritability 42.9 19.8 1.1 34.9 17.4 4.7 Table 3: Frequency of Solicited Injection Site and Systemic Adverse Reactions Within 7 Days After Vaccination with Fluzone, Children 3 Through 8 Years of Age (Study 1*) Dose 1 (N†=150–151)
PercentageDose 2 (N†=144–145)
PercentageAny Moderate‡ Severe§ Any Moderate‡ Severe§ "-" Indicates information was not collected - * (NCT00391391)
- † N is the number of vaccinated participants with available data for the adverse reactions listed
- ‡ Moderate - Injection-site pain: sufficiently discomforting to interfere with normal behavior or activities; Injection-site erythema, Injection-site swelling, Injection-site induration, and Injection-site ecchymosis: ≥2.5 cm to <5 cm; Fever: >100.4°F to ≤102.2°F; Headache, Malaise, and Myalgia: interferes with daily activities
- § Severe - Injection-site pain: incapacitating, unable to perform usual activities, may have/or required medical care or absenteeism; Injection-site erythema, Injection-site swelling, Injection-site induration, and Injection-site ecchymosis: ≥5 cm; Fever: >102.2°F; Headache, Malaise, and Myalgia: prevents daily activities
- ¶ Fever - The percentage of temperature measurements that were taken by oral or axillary routes, or not recorded were 93.4%, 6.6%, and 0.0%, respectively, for Dose 1; and 93.1%, 6.2%, and 0.7%, respectively, for Dose 2
Injection-Site Pain 59.3 8.0 0.0 62.1 9.7 0.7 Injection-Site Erythema 27.8 3.3 0.7 27.6 2.1 0.7 Injection-Site Swelling 19.9 5.3 0.0 14.5 2.8 0.0 Injection-Site Induration 16.6 2.0 0.0 11.7 1.4 0.0 Injection-Site Ecchymosis 12.6 0.7 0.7 15.2 0.7 0.0 Injection-Site Pruritus 7.3 - - 13.2 - - Fever¶ (≥99.5°F) 11.9 2.6 2.0 9.7 1.4 1.4 Headache 16.7 2.0 0.7 11.8 1.4 1.4 Malaise 20.0 2.7 1.3 14.6 4.2 0.7 Myalgia 28.0 5.3 0.0 17.4 4.2 0.0 During the period from the first vaccination through 6 months following the second vaccination, there were no serious adverse events considered to be caused by vaccination and no deaths reported in this study.
Study 2 (NCT01240746) was a single-blind, randomized, active-controlled multi-center safety and immunogenicity study conducted in the US. In this study, children 6 months through 35 months of age received one or two 0.25 mL doses of either Fluzone Quadrivalent or one of two formulations of a comparator trivalent influenza vaccine (TIV-1 or TIV-2), and children 3 years through 8 years of age received one or two 0.5 mL doses of either Fluzone Quadrivalent, TIV-1, or TIV-2. Each of the trivalent formulations contained an influenza type B virus that corresponded to one of the two type B viruses in Fluzone Quadrivalent (a type B virus of the Victoria lineage or a type B virus of the Yamagata lineage). For participants who received two doses, the doses were administered approximately 4 weeks apart. The safety analysis set included 1841 children 6 months through 35 months of age and 2506 children 3 years through 8 years of age. Among participants 6 months through 8 years of age in the three vaccine groups combined, 49.3% were female (Fluzone Quadrivalent, 49.2%; TIV-1, 49.8%; TIV-2, 49.4%), 58.4% Caucasian (Fluzone Quadrivalent, 58.4%; TIV-1, 58.9%; TIV-2, 57.8%), 20.2% Black (Fluzone Quadrivalent, 20.5%; TIV-1, 19.9%; TIV-2, 19.1%), 14.1% Hispanic (Fluzone Quadrivalent, 14.3%; TIV-1, 13.2%; TIV-2, 14.7%), and 7.3% were of other racial/ethnic groups (Fluzone Quadrivalent, 6.8%; TIV-1, 8.0%; TIV-2, 8.5%). Table 4 and Table 5 summarize solicited injection-site and systemic adverse reactions reported within 7 days post-vaccination via diary cards. Participants were monitored for unsolicited adverse events for 28 days after each dose and serious adverse events (SAEs) during the 6 months following the last dose.
Table 4: Percentage of Solicited Injection-site and Systemic Adverse Reactions Within 7 Days After Vaccination in Children 6 Months Through 35 Months of Age (Study 2* Safety Analysis Set†) Fluzone Quadrivalent‡,§
(N¶=1223)TIV-1§,#
(B Victoria)
(N¶=310)TIV-2§,Þ
(B Yamagata)
(N¶=308)Any
(%)Grade 2ß
(%)Grade 3à
(%)Any
(%)Grade 2ß
(%)Grade 3à
(%)Any
(%)Grade 2ß
(%)Grade 3à
(%)- * NCT01240746
- † The safety analysis set includes all persons who received at least one dose of study vaccine
- ‡ Fluzone Quadrivalent (0.25 mL) containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), B/Brisbane/60/2008 (Victoria lineage), and B/Florida/04/2006 (Yamagata lineage)
- § Participants received 1 or 2 doses according to ACIP recommendations
- ¶ N is the number of participants in the safety analysis set
- # 2010-2011 Fluzone TIV (0.25 mL) containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Brisbane/60/2008 (Victoria lineage), licensed
- Þ Investigational TIV (0.25 mL) containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Florida/04/2006 (Yamagata lineage), non-licensed
- ß Grade 2 - Injection-site pain: sufficiently discomforting to interfere with normal behavior or activities; Injection-site tenderness: cries and protests when injection-site is touched; Injection-site erythema, Injection-site swelling: ≥2.5 cm to <5 cm; Fever: >101.3°F to ≤103.1°F (6 months through 23 months); ≥101.2°F to ≤102.0°F (24 months through 35 months); Malaise, Myalgia, and Headache: some interference with activity; Irritability: requiring increased attention; Crying abnormal: 1 to 3 hours; Drowsiness: not interested in surroundings or did not wake up for a feed/meal; Appetite loss: missed 1 or 2 feeds/meals completely; Vomiting: 2 to 5 episodes per 24 hours
- à Grade 3 - Injection-site pain: incapacitating, unable to perform usual activities; Injection-site tenderness: cries when injected limb is moved, or the movement of the injected limb is reduced; Injection-site erythema, Injection-site swelling: ≥5 cm; Fever: >103.1°F (6 months through 23 months); ≥102.1°F (24 months through 35 months); Malaise, Myalgia, and Headache: Significant; prevents daily activity; Irritability: inconsolable; Crying abnormal: >3 hours; Drowsiness: sleeping most of the time or difficult to wake up; Appetite loss: refuses ≥3 feeds/meals or refuses most feeds/meals; Vomiting: ≥6 episodes per 24 hours or requiring parenteral hydration
- è Assessed in children 24 months through 35 months of age
- ð Assessed in children 6 months through 23 months of age
- ø Fever measured by any route
Injection-site adverse reactions Painè 57.0 10.2 1.0 52.3 11.5 0.8 50.3 5.4 2.7 Tendernessð 54.1 11.3 1.9 48.4 8.2 1.9 49.7 10.3 0.0 Erythema 37.3 1.5 0.2 32.9 1.0 0.0 33.3 1.0 0.0 Swelling 21.6 0.8 0.2 19.7 1.0 0.0 17.3 0.0 0.0 Systemic adverse reactions Fever (≥100.4°F)ø 14.3 5.5 2.1 16.0 6.6 1.7 13.0 4.1 2.0 Malaiseè 38.1 14.5 4.6 35.2 14.8 4.7 32.4 12.8 6.8 Myalgiaè 26.7 6.6 1.9 26.6 9.4 1.6 25.0 6.8 2.7 Headacheè 8.9 2.5 0.6 9.4 3.9 0.0 12.2 4.7 0.0 Irritabilityð 54.0 26.4 3.2 52.8 20.1 3.1 53.5 22.9 2.8 Crying abnormalð 41.2 12.3 3.3 36.5 8.2 1.9 29.9 10.4 2.1 Drowsinessð 37.7 8.4 1.3 32.1 3.8 0.6 31.9 5.6 0.7 Appetite lossð 32.3 9.1 1.8 33.3 5.7 1.9 25.0 8.3 0.7 Vomitingð 14.8 6.2 1.0 11.3 4.4 0.6 13.9 6.3 0.0 Table 5: Percentage of Solicited Injection-site and Systemic Adverse Reactions Within 7 Days After Vaccination in Children 3 Years Through 8 Years of Age (Study 2* Safety Analysis Set†) Fluzone Quadrivalent‡
(N§=1669)TIV-1¶
(B Victoria)
(N§=424)TIV-2#
(B Yamagata)
(N§=413)Any
(%)Grade 2Þ
(%)Grade 3ß
(%)Any
(%)Grade 2Þ
(%)Grade 3ß
(%)Any
(%)Grade 2Þ
(%)Grade 3ß
(%)- * NCT01240746
- † The safety analysis set includes all persons who received at least one dose of study vaccine
- ‡ Fluzone Quadrivalent containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), B/Brisbane/60/2008 (Victoria lineage), and B/Florida/04/2006 (Yamagata lineage)
- § N is the number of participants in the safety analysis set
- ¶ 2010-2011 Fluzone TIV containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Brisbane/60/2008 (Victoria lineage), licensed
- # Investigational TIV containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Florida/04/2006 (Yamagata lineage), non-licensed
- Þ Grade 2 - Injection-site pain: sufficiently discomforting to interfere with normal behavior or activities; Injection-site erythema, Injection-site swelling: ≥2.5 cm to <5 cm; Fever: ≥101.2°F to ≤102.0°F; Headache, Malaise, and Myalgia: some interference with activity
- ß Grade 3 - Injection-site pain: incapacitating, unable to perform usual activities; Injection-site erythema, Injection-site swelling: ≥5 cm; Fever: ≥102.1°F; Headache, Malaise, and Myalgia: Significant; prevents daily activity
- à Fever measured by any route
Injection-site adverse reactions Pain 66.6 15.8 2.1 64.6 9.5 2.0 63.8 11.6 2.8 Erythema 34.1 2.9 1.8 36.8 3.4 1.2 35.2 2.5 1.8 Swelling 24.8 2.8 1.4 25.4 1.5 1.2 25.9 2.5 1.8 Systemic adverse reactions Fever (≥100.4°F)à 7.0 2.1 2.1 7.1 2.2 1.2 7.6 2.8 0.8 Headache 23.1 6.8 2.2 21.2 5.1 2.7 24.4 7.5 2.0 Malaise 31.9 11.2 5.5 32.8 11.4 5.6 33.4 10.8 5.0 Myalgia 38.6 12.2 3.3 34.1 9.0 2.7 38.4 11.1 2.8 Among children 6 months through 8 years of age, unsolicited non-serious adverse events were reported in 1360 (47.0%) recipients in the Fluzone Quadrivalent group, 352 (48.0%) recipients in the TIV-1 group, and 346 (48.0%) recipients in the TIV-2 group. The most commonly reported unsolicited non-serious adverse events were cough, vomiting, and pyrexia. During the 28 days following vaccination, a total of 16 (0.6%) recipients in the Fluzone Quadrivalent group, 4 (0.5%) recipients in the TIV-1 group, and 4 (0.6%) recipients in the TIV-2 group, experienced at least one SAE. Throughout the study period, a total of 41 (1.4%) recipients in the Fluzone Quadrivalent group, 7 (1.0%) recipients in the TIV-1 group, and 14 (1.9%) recipients in the TIV-2 group, experienced at least one SAE. Three SAEs were considered to be possibly related to vaccination: croup in a Fluzone Quadrivalent recipient and 2 episodes of febrile seizure, 1 each in a TIV-1 recipient and a TIV-2 recipient.
Study 3 (NCT02915302) was a randomized, observer-blinded, 2-arm, multi-center safety and immunogenicity study conducted in the US. In this study, 1950 children 6 months through 35 months of age were randomly assigned to receive Fluzone Quadrivalent administered in either a volume of 0.25 mL (Group 1) or 0.5 mL (Group 2). For participants recommended to receive two doses of influenza vaccine as per Advisory Committee on Immunization Practices guidance, the same dose was administered 4 weeks after the first. The safety analysis set included 1941 participants who received at least 1 dose of study vaccine. Of these participants, 49.7% were female, 74.3% were Caucasian, 19.2% were Black, 6.5% were of other racial groups, and 22.0% were Hispanic/Latino. Data for Fluzone Quadrivalent are relevant to Fluzone because both vaccines are manufactured using the same process and have overlapping compositions.
Table 6 summarizes solicited injection-site and systemic adverse reactions reported within 7 days post-vaccination via diary cards for the 0.25 mL and 0.5 mL volumes of Fluzone Quadrivalent in children 6 months through 35 months of age.
Table 6: Percentage of Solicited Injection Site and Systemic Adverse Reactions Within 7 Days After Vaccination in Children 6 Months Through 35 Month of Age (Study 3* Safety Analysis Set†) Fluzone Quadrivalent
0.25 mL‡
(N§=949)Fluzone Quadrivalent
0.5 mL‡
(N§=992)Any
(%)Grade 3¶
(%)Any
(%)Grade 3¶
(%)- * NCT02915302
- † The safety analysis set includes all persons who received at least one dose of study vaccine
- ‡ Participants received 1 or 2 doses according to ACIP recommendations
- § N is the number of participants in the safety analysis set
- ¶ Grade 3 - Injection-site tenderness: Cries when injected limb is moved, or the movement of the injected limb is reduced; Injection-site redness, Injection-site swelling: ≥ 50 mm; Irritability: inconsolable; Abnormal Crying: > 3 hours; Drowsiness: sleeping most of the time or difficult to wake up; Loss of Appetite: refuses ≥ 3 feeds/meals or refuses most feeds/meals; Fever: >103.1°F; Vomiting: ≥ 6 episodes per 24 hours or requiring parenteral hydration
- # Fever measured by any route
Injection-site adverse reactions Tenderness 47.3 1.7 50.4 1.2 Redness 23.1 0.0 24.3 0.2 Swelling 12.9 0.1 14.7 0.0 Systemic adverse reactions Irritability 47.4 3.6 48.6 4.0 Abnormal Crying 33.3 3.1 34.1 2.6 Drowsiness 31.9 2.1 31.3 1.6 Loss of Appetite 27.3 1.4 28.3 2.2 Fever (≥100.4°F) # 11.3 0.6 12.2 1.2 Vomiting 10.0 0.4 10.2 0.5 The difference in fever rate (Group 2 minus Group 1) was 0.84% (95% CI: -2.13%; 3.80%), meeting the prespecified non-inferiority criterion (upper limit of the 2-sided 95% CI of the difference in fever rates < 5%). Participants were monitored for unsolicited adverse events and SAEs during the 28 days following vaccination. Unsolicited non-serious adverse events were reported in 417 (44%) participants in Group 1 and 394 (40%) participants in Group 2. The most commonly reported unsolicited non-serious adverse events in both groups were cough and rhinorrhea. Ten SAEs were reported during the 28-day follow-up period: 5 (0.5%) in Group 1 and 5 (0.5%) in Group 2.
Adults
Study 4 (NCT00772109) was a multi-center trial conducted in the US. In this study adults 18 through 64 years of age received Fluzone (2008–2009 formulation). The safety analysis set included 1421 Fluzone recipients. Table 7 summarizes solicited injection-site reactions and systemic adverse reactions reported within 7 days post-vaccination via diary cards.
Table 7: Frequency of Solicited Injection Site and Systemic Adverse Reactions Within 7 Days After Vaccination with Fluzone, Adults 18 Through 64 Years of Age (Study 4*) (N†=1392–1394)
PercentageAny Grade 2‡ Grade 3§ - * NCT00772109
- † N is the number of vaccinated participants with available data for the adverse reactions listed
- ‡ Grade 2 - Injection-site erythema, Injection-site induration, Injection-site swelling, and Injection-site ecchymosis: ≥2.5 cm to <5 cm; Injection-site pain and Injection-site pruritus: sufficiently discomforting to interfere with normal behavior or activities; Fever: >100.4°F to ≤102.2°F; Headache, Myalgia, Malaise, and Shivering: interferes with daily activities
- § Grade 3 - Injection-site erythema, Injection-site induration, Injection-site swelling, and Injection-site ecchymosis: ≥5 cm; Injection-site pain: incapacitating, unable to perform usual activities; Injection-site pruritus: incapacitating, unable to perform usual activities, may have/or required medical care or absenteeism; Fever: >102.2°F; Headache, Myalgia, Malaise, and Shivering: prevents daily activities
- ¶ Fever - The percentage of temperature measurements that were taken by oral or axillary routes, or not recorded were 99.6%, 0.0%, and 0.4%, respectively
Injection-Site Erythema 13.2 2.1 0.9 Injection-Site Induration 10.0 2.3 0.5 Injection-Site Swelling 8.4 2.1 0.9 Injection-Site Pain 53.7 5.8 0.8 Injection-Site Pruritus 9.3 0.4 0.0 Injection-Site Ecchymosis 6.2 1.1 0.4 Headache 30.3 6.5 1.6 Myalgia 30.8 5.5 1.4 Malaise 22.2 5.5 1.8 Shivering 6.2 1.1 0.6 Fever¶ (≥99.5°F) 2.6 0.4 0.2 Within 28 days and 6 months post-vaccination, a serious adverse event was reported by 5 (0.4%) and 20 (1.4%) Fluzone recipients, respectively. No serious adverse event was considered to be caused by vaccination. No deaths were reported during the 6 months post-vaccination.
In Study 5 (NCT00391053) adults 65 years of age and older received Fluzone (2006–2007 formulation). The study was a multi-center, double-blind trial conducted in the US. The safety analysis set included 1260 Fluzone recipients.
Table 8 summarizes solicited injection-site reactions and systemic adverse reactions reported within 7 days post-vaccination via diary cards. Onset was usually within the first 3 days after vaccination and a majority of the adverse reactions resolved within 3 days.
Table 8: Frequency of Solicited Injection Site and Systemic Adverse Reactions Within 7 Days After Vaccination with Fluzone, Adults 65 Years of Age and Older (Study 5*) N†=1258–1260
PercentageAny Moderate‡ Severe§ - * NCT00391053
- † N is the number of vaccinated participants with available data for the adverse reactions listed
- ‡ Moderate - Injection-site pain: sufficiently discomforting to interfere with normal behavior or activities; Injection-site erythema and Injection-site swelling: ≥2.5 cm to <5 cm; Fever: >100.4°F to ≤102.2°F; Myalgia, Malaise, and Headache: interferes with daily activities
- § Severe - Injection-site pain: incapacitating, unable to perform usual activities; Injection-site erythema and Injection-site swelling: ≥5 cm; Fever: >102.2°F; Myalgia, Malaise, and Headache: prevents daily activities
- ¶ Fever - The percentage of temperature measurements that were taken by oral route or not recorded were 98.6% and 1.4%, respectively
Injection-Site Pain 24.3 1.7 0.2 Injection-Site Erythema 10.8 0.8 0.6 Injection-Site Swelling 5.8 1.3 0.6 Myalgia 18.3 3.2 0.2 Malaise 14.0 3.7 0.6 Headache 14.4 2.5 0.3 Fever¶ (≥99.5°F) 2.3 0.2 0.1 Within 6 months post-vaccination, 93 (7.4%) Fluzone recipients experienced a serious adverse event (N=1260). No deaths were reported within 28 days post-vaccination. A total of 7 deaths were reported during the period Day 29–180 post-vaccination: 7 (0.6%) among Fluzone recipients (N=1260). The majority of these participants had a medical history of cardiac, hepatic, neoplastic, renal, and/or respiratory diseases. No deaths were considered to be caused by vaccination.
6.2 Postmarketing Experience
The following adverse events have been spontaneously reported during the post-approval use of Fluzone or Fluzone Quadrivalent. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to Fluzone or Fluzone Quadrivalent.
- Blood and Lymphatic System Disorders: Thrombocytopenia, lymphadenopathy
- Immune System Disorders: Anaphylaxis, other allergic/hypersensitivity reactions (including urticaria, angioedema)
- Eye Disorders: Ocular hyperemia
- Nervous System Disorders: Guillain-Barré syndrome (GBS), convulsions, febrile convulsions, myelitis (including encephalomyelitis and transverse myelitis), facial palsy (Bell's palsy), optic neuritis/neuropathy, brachial neuritis, syncope (shortly after vaccination), dizziness, paresthesia
- Vascular Disorders: Vasculitis, vasodilatation/flushing
- Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, oropharyngeal pain, rhinorrhea, cough, wheezing, throat tightness
- Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
- General Disorders and Administration Site Conditions: Pruritus, asthenia/fatigue, pain in extremities, chest pain
- Gastrointestinal Disorders: Vomiting
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Available data with Fluzone use in pregnant women are insufficient to inform vaccine-associated risk of adverse developmental outcomes.
There were no developmental studies of Fluzone performed in animals. The developmental effects of Fluzone Quadrivalent are relevant to Fluzone because both vaccines are manufactured using the same process and have overlapping compositions. A developmental toxicity study was performed in female rabbits administered Fluzone Quadrivalent prior to mating and during gestation. The dose was 0.5 mL on each of five occasions (a single human dose is 0.5 mL). This study revealed no adverse effects to the fetus or pre-weaning development and no evidence of impaired female fertility due to Fluzone Quadrivalent (see Data).
Data
Animal Data: A developmental toxicity study was performed in female rabbits administered Fluzone Quadrivalent by intramuscular injection on 24 and 10 days before insemination, and on Days 6, 12, and 27 of gestation. The dose was 0.5 mL on each occasion (a single human dose is 0.5 mL). This study revealed no vaccine related fetal malformations and no adverse effects on pre-weaning development or female fertility.
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk
Pregnant women are at increased risk of complications associated with influenza infection compared to non-pregnant women. Pregnant women who contract influenza may be at increased risk for adverse pregnancy outcomes, including preterm labor and delivery.
8.2 Lactation
It is not known if Fluzone is excreted in human milk. Data are not available to assess the effects of Fluzone on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Fluzone and any potential adverse effects on the breastfed child from Fluzone or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to the disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness of Fluzone in children below the age of 6 months have not been established. Safety and effectiveness of Fluzone in children 9 through 17 years of age is based on safety and effectiveness in children 6 months through 8 years of age and adults 18 years of age and older.
8.5 Geriatric Use
Safety and immunogenicity of Fluzone were evaluated in adults 65 years of age and older. [See Adverse Reactions (6.1) and Clinical Studies (14.3)] Antibody responses to Fluzone are lower in persons ≥65 years of age than in younger adults. [See Clinical Studies (14.5, 14.6)]
-
11 DESCRIPTION
Fluzone (Influenza Vaccine) for intramuscular use is an inactivated influenza vaccine, prepared from influenza viruses propagated in embryonated chicken eggs. The virus-containing allantoic fluid is harvested and inactivated with formaldehyde. Influenza virus is concentrated and purified in a linear sucrose density gradient solution using a continuous flow centrifuge. The virus is then chemically disrupted using a non-ionic surfactant, octylphenol ethoxylate (Triton® X-100), producing a "split virus". The split virus containing hemagglutinin (HA) antigen is further purified and then suspended in sodium phosphate-buffered isotonic sodium chloride solution. The purified split virus from the three strains included in the vaccine are produced separately and then combined to make the trivalent formulation.
Fluzone is an injectable suspension and is clear and slightly opalescent in color.
Antibiotics are not used in the manufacture of Fluzone.
No presentation of Fluzone is made with natural rubber latex.
Fluzone is standardized according to United States Public Health Service requirements and is formulated to contain HA of each of the following three influenza strains recommended for the 2025-2026 influenza season: A/Victoria/4897/2022 IVR-238 (H1N1), A/Croatia/10136RV/2023 X-425A (H3N2), and B/Michigan/01/2021 (a B/Austria/1359417/2021-like virus, B Victoria lineage). The amounts of HA and other ingredients per dose of vaccine are listed in Table 9. The 0.5 mL single-dose, pre-filled syringe presentation is manufactured and formulated without thimerosal or any other preservative. The 5 mL multi-dose vial presentation contains thimerosal, a mercury derivative, added as a preservative. Each 0.5 mL dose from the multi-dose vial contains 25 mcg mercury. Each 0.25 mL dose from the multi-dose vial contains 12.5 mcg mercury.
Table 9: Fluzone Ingredients Ingredient Quantity
(per dose)Fluzone
0.25 mL DoseFluzone
0.5 mL Dose"-" Indicates information is not applicable - * per United States Public Health Service (USPHS) requirement
- † Quantity Sufficient
Active Substance: Split influenza virus, inactivated strains*: 22.5 mcg HA total 45 mcg HA total A (H1N1) 7.5 mcg HA 15 mcg HA A (H3N2) 7.5 mcg HA 15 mcg HA B 7.5 mcg HA 15 mcg HA Other: Sodium phosphate-buffered isotonic sodium chloride solution QS† to appropriate volume QS† to appropriate volume Formaldehyde ≤50 mcg ≤100 mcg Octylphenol ethoxylate ≤125 mcg ≤250 mcg Preservative Single-dose presentations - - Multi-dose presentation (thimerosal) 12.5 mcg mercury 25 mcg mercury -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Specific levels of hemagglutination inhibition (HI) antibody titer post-vaccination with inactivated influenza virus vaccines have not been correlated with protection from influenza virus infection. In some human studies, antibody titers ≥1:40 have been associated with protection from influenza illness in up to 50% of participants. (2) (3)
Antibodies against one influenza virus type or subtype confer limited or no protection against another. Furthermore, antibodies to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change of one or more new strains in each year's influenza vaccine.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Efficacy of Fluzone in Children 6 through 24 Months of Age
Study 6 (NCT not available) was a randomized, double-blind, placebo-controlled study conducted at a single US center during the 1999–2000 (Year 1) and 2000–2001 (Year 2) influenza seasons. The intent-to-treat analysis set included a total of 786 children 6 through 24 months of age. Participants received two 0.25ml doses of either Fluzone (N = 525) or a placebo (N = 261) 4 weeks apart. Among all randomized participants in both years, the mean age was 13.8 months; 52.5% were male, 50.8% were Caucasian, 42.0% were Black, and 7.2% were of other racial groups. Cases of influenza were identified through active and passive surveillance for influenza-like illness or acute otitis media and confirmed by culture. Influenza-like illness was defined as fever with signs or symptoms of an upper respiratory infection. Vaccine efficacy against all influenza viral types and subtypes was a secondary endpoint and is presented in Table 10.
Table 10: Estimated Efficacy of Fluzone Against Culture-Confirmed Influenza in Children Aged 6 through 24 Months during the 1999–2000 and 2000–2001 Influenza Seasons – Intent-to-Treat Analysis Set* (Study 6) Fluzone† Placebo‡ Fluzone vs. Placebo Year n§ N¶ Rate (n/N) # (95% CI) n§ N¶ Rate
(n/N) #(95% CI) Relative Risk
(95% CI)Percent Relative ReductionÞ
(95% CI)- * The intent-to-treat analysis set includes all enrolled participants who were randomly assigned to receive Fluzone or placebo and vaccinated
- † Fluzone: 1999–2000 formulation containing A/Beijing/262/95 (H1N1), A/Sydney/15/97 (H3N2), and B/Yamanashi/166/98 (Yamagata lineage) and 2000–2001 formulation containing A/New Caledonia/20/99 (H1N1), A/Panama/2007/99 (H3N2), and B/Yamanashi/166/98 (Yamagata lineage)
- ‡ Placebo: 0.4% NaCl
- § n is the number of participants with culture-confirmed influenza for the given year of study as listed in the first column
- ¶ N is the number of participants randomly assigned to receive Fluzone or placebo for the given year of study as listed in the column headers (intent-to-treat analysis set)
- # Rate (%) = (n/N) * 100
- Þ Relative reduction in vaccine efficacy was defined as (1-relative risk) × 100
- ß Includes all culture confirmed influenza cases throughout the study duration for Year 1 (12 months of follow-up)
- à Includes all culture-confirmed influenza cases throughout the study duration for Year 2 (6 months of follow-up)
Year 1ß
(1999–2000)15 273 5.5 (3.1; 8.9) 22 138 15.9 (10.3; 23.1) 0.34 (0.18; 0.64) 66 (36; 82) Year 2 à
(2000–2001)9 252 3.6 (1.6; 6.7) 4 123 3.3 (0.9; 8.1) 1.10 (0.34; 3.50) -10 (-250; 66) 14.2 Efficacy of Fluzone in Adults
Study 7 (NCT00538512) was a randomized, double-blind, placebo-controlled study conducted in a single US center during the 2007–2008 influenza season. Participants received one dose of either Fluzone vaccine (N = 813), an active comparator (N = 814), or placebo (N = 325). The intent-to-treat analysis set included 1138 healthy adults who received Fluzone or placebo. Participants were 18 through 49 years of age (mean age was 23.3 years); 63.3% were female, 83.1% were Caucasian, and 16.9% were of other racial/ethnic groups. Cases of influenza were identified through active and passive surveillance and confirmed by cell culture and/or real-time polymerase chain reaction (PCR). Influenza-like illness was defined as an illness with at least 1 respiratory symptom (cough or nasal congestion) and at least 1 constitutional symptom (fever or feverishness, chills, or body aches). Vaccine efficacy of Fluzone against all influenza viral types and subtypes is presented in Table 11.
Table 11: Estimated Efficacy of Fluzone Vaccine Against Influenza in Adults Aged 18 through 49 Years during the 2007–2008 Influenza Season – Intent-to-Treat Analysis Set* (Study 7†) Laboratory-Confirmed Symptomatic Influenza Fluzone‡
(N=813) §Placebo¶
(N=325) §Fluzone vs. Placebo n# Rate
(%)Þ(95% CI) n# Rate
(%)Þ(95% CI) Relative Risk
(95% CI)Percent Relative Reductionß
(95% CI)- * The intent-to-treat analysis set includes all enrolled participants who were randomly assigned to receive Fluzone or placebo and vaccinated
- † NCT00538512
- ‡ Fluzone: 2007–2008 formulation containing A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 (Victoria lineage)
- § N is the number of participants randomly assigned to receive Fluzone or placebo
- ¶ Placebo: 0.9% NaCl
- # n is the number of participants satisfying the criteria listed in the first column
- Þ Rate (%) = (n/N) * 100
- ß Relative reduction in vaccine efficacy was defined as (1 - relative risk) × 100
Positive culture 21 2.6 (1.6; 3.9) 31 9.5 (6.6; 13.3) 0.27 (0.16; 0.46) 73 (54; 84) Positive PCR 28 3.4 (2.3; 4.9) 35 10.8 (7.6; 14.7) 0.32 (0.20; 0.52) 68 (48; 80) Positive culture, positive PCR, or both 28 3.4 (2.3; 4.9) 35 10.8 (7.6; 14.7) 0.32 (0.20; 0.52) 68 (48; 80) 14.3 Immunogenicity of Fluzone in Children 6 Months through 8 Years of Age
In Study 1, a multi-center study conducted in the US, 68 children 6 months through 35 months of age given two 0.25 mL doses of Fluzone and 120 children 3 years through 8 years of age given two 0.5 mL doses of Fluzone were included in the per-protocol analysis set. The two doses (2006–2007 formulation) were administered 26 to 30 days apart. Females accounted for 42.6% of the participants in the 6 months through 35 months age group and 53.3% of the participants in the 3 years through 8 years age group. Most participants in the 6 months through 35 months and 3 years through 8 years age groups, respectively, were Caucasian (70.6% and 79.2%), followed by Hispanic (19.1% and 13.3%), and Black (7.4% and 4.2%).
The percentage of participants who received influenza vaccination during the previous influenza season was 54.4% for the 6 months through 35 months age group and 27.5% for the 3 years through 8 years age group. Table 12 shows seroconversion rates and the percentage of participants with an HI titer ≥1:40 pre-vaccination and one month following the second dose of Fluzone.
Table 12: Percentage (%) with Pre and Post-Vaccination HI Titers ≥1:40 and Seroconversion Following the Second Vaccine Injection with Fluzone* in Children 6 Months Through 35 Months and 3 Years Through 8 Years of Age (Study 1†) Antigen Age Group Pre-Vaccination Titer ≥1:40
% (95% CI)Post-Vaccination‡ Titer ≥1:40
% (95% CI)Seroconversion§
% (95% CI)N=68 (6 to 35 months); N=120 (3 through 8 years) - * Children received two doses of Fluzone administered 26 to 30 days apart, irrespective of previous influenza vaccination history
- † NCT00391391
- ‡ Post-vaccination HI titers drawn at 28 days post-dose
- § Seroconversion: Paired samples with pre-vaccination HI titer <1:10 and post-vaccination (28 days post-dose 2) titer ≥1:40 or a minimum 4-fold increase for participants with pre-vaccination titer ≥1:10
A (H1N1) 6 through 35 months 11.8 (5.2; 21.9) 92.6 (83.7; 97.6) 88.2 (78.1; 94.8) 3 through 8 years 40.0 (31.2; 49.3) 99.2 (95.4; 100.0) 78.3 (69.9; 85.3) A (H3N2) 6 through 35 months 29.4 (19.0; 41.7) 100.0 (94.7; 100.0) 91.2 (81.8; 96.7) 3 through 8 years 80.0 (71.7; 86.7) 100.0 (97.0; 100.0) 61.7 (52.4; 70.4) B 6 through 35 months 1.5 (0.0; 7.9) 20.6 (11.7; 32.1) 20.6 (11.7; 32.1) 3 through 8 years 3.3 (0.9; 8.3) 58.3 (49.0; 67.3) 53.3 (44.0; 62.5) 14.4 Immunogenicity of a 0.5 mL Dose of Fluzone Quadrivalent in Children 6 Months through 35 Months of Age
In Study 3 (NCT02915302) [see Adverse Reactions (6.1)], 1027 children, 6 months through 35 months of age, were included in the per-protocol immunogenicity analysis. The distribution of demographic characteristics was similar to that of the safety analysis set [see Adverse Reactions (6.1)].
In this study, children 6 months through 35 months of age received one or two doses of either 0.25 mL or 0.5 mL of Fluzone Quadrivalent. Non-inferiority of the 0.5 mL dose(s) relative to the 0.25 mL dose(s) of Fluzone Quadrivalent was demonstrated for all four strains based on pre- specified criteria (lower limit of the 2-sided 95% CI of the ratio of GMTs between groups >0.667; lower limit of the 2-sided 95% CI of the difference in seroconversion rates >-10%). GMT ratios (GMT0.5-mL dose divided by GMT0.25-mL dose) for the A/H1N1, A/H3N2, B Victoria lineage, and B Yamagata lineage strains were 1.42 (95% CI: 1.16; 1.74), 1.48 (95% CI: 1.21; 1.82), 1.33 (95% CI: 1.09; 1.62), and 1.41 (95% CI: 1.17; 1.70), respectively. Seroconversion rate (SCR) differences (SCR0.5-mL dose minus SCR0.25-mL dose) for the A/H1N1, A/H3N2, B Victoria lineage, and B Yamagata lineage strains were 4.6% (95% CI: -0.4%; 9.6%), 5.1% (95% CI: 0.4%; 9.8%), 1.3% (95% CI: -2.9%; 5.6%), and 2.6% (95% CI: -1.4%; 6.5%). Data for Fluzone Quadrivalent are relevant to Fluzone because both vaccines are manufactured using the same process and have overlapping compositions.
14.5 Immunogenicity of Fluzone in Adults
Adults 18 through 64 years of age received Fluzone (2008–2009 formulation) in Study 4, a multi-center trial conducted in the US. For immunogenicity analyses, there were 1287 participants who received Fluzone in the per-protocol analysis set. There were fewer males (35.8%) than females. The mean age was 42.6 years (ranged from 18.2 through 65.0 years). Most participants were Caucasian (80.0%), followed by Hispanic (11.0%), and Black (6.3%). Table 13 shows seroconversion rates at 28 days following vaccination and the percentage of participants with an HI titer ≥1:40 prior to vaccination and 28 days following vaccination.
Table 13: Percentage (%) with Pre and Post-Vaccination HI Titers ≥1:40 and Seroconversion in Adult Fluzone Recipients 18 Through 64 Years of Age (Study 4*) Antigen Pre-Vaccination Titer ≥1:40 Post-Vaccination† Titer ≥1:40 Seroconversion‡ % (95% CI)
N§=1285–1286% (95% CI)
N§=1283–1285% (95% CI)
N§=1283–1285- * NCT00772109
- † Post-vaccination HI titers drawn at 28 days post-dose
- ‡ Seroconversion: Paired samples with pre-vaccination HI titer <1:10 and post-vaccination (28 days post-dose) titer ≥1:40 or a minimum 4-fold increase for participants with pre-vaccination titer ≥1:10
- § N is the number of vaccinated participants with available data for the immunologic endpoint listed
A (H1N1) 39.1 (36.4; 41.8) 91.7 (90.0; 93.1) 60.5 (57.7; 63.2) A (H3N2) 33.6 (31.0; 36.2) 91.4 (89.8; 92.9) 74.8 (72.3; 77.1) B 41.2 (38.5; 44.0) 89.3 (87.4; 90.9) 54.2 (51.4; 56.9) 14.6 Immunogenicity of Fluzone in Geriatric Adults
Adults 65 years of age and older received Fluzone (2006–2007 formulation) in Study 5, a multi-center trial conducted in the US. For immunogenicity analyses, there were 1275 participants who received Fluzone in the immunogenicity analysis set. Females accounted for 54.7% of participants. The mean age was 72.9 years (ranged from 65 through 94 years of age); 36% of participants were 75 years of age or older. Most participants were Caucasian (92.9%), followed by Hispanic (3.7%), and Black (2.7%). Table 14 shows seroconversion rates at 28 days following vaccination and the percentage of participants with an HI titer ≥1:40 prior to vaccination and 28 days following vaccination.
Table 14: Percentage (%) with Pre and Post-Vaccination HI Titers ≥1:40 and Seroconversion in Adult Fluzone Recipients 65 Years of Age and Older (Study 5*) Antigen Pre-Vaccination HI Titer ≥1:40 Post-Vaccination† Titer ≥1:40 Seroconversion‡ % (95% CI)
N§=1267–1268% (95% CI)
N§=1252% (95% CI)
N§=1248–1249- * NCT00391053
- † Post-vaccination HI titers drawn at 28 days post-dose
- ‡ Seroconversion: Paired samples with pre-vaccination HI titer <1:10 and post-vaccination (28 days post-dose) titer ≥1:40 or a minimum 4-fold increase for participants with pre-vaccination titer ≥1:10
- § N is the number of vaccinated participants with available data for the immunologic endpoint listed
A (H1N1) 45.9 (43.2; 48.7) 76.8 (74.3; 79.1) 23.1 (20.8; 25.6) A (H3N2) 68.6 (66.0; 71.2) 96.5 (95.3; 97.4) 50.7 (47.9; 53.5) B 27.3 (24.9; 29.9) 67.6 (64.9; 70.2) 29.9 (27.4; 32.6) -
15 REFERENCES
- 1 Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992–1993 and 1993–1994 influenza vaccines. N Engl J Med 1998;339:1797–802.
- 2 Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004;103:133–138.
- 3 Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972;70:767–777.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Multi-dose vial, 5 mL (NDC: 49281-643-78) (not made with natural rubber latex). Supplied as package of one (NDC: 49281-643-15). A maximum of ten doses can be withdrawn from the multi-dose vial.
Single-dose, prefilled syringe (clear plunger rod), without needle, 0.5 mL (NDC: 49281-425-88) (not made with natural rubber latex). Supplied as package of 10 (NDC: 49281-425-50).
16.2 Storage and Handling
Store all Fluzone presentations refrigerated at 2° to 8°C (35° to 46°F). DO NOT FREEZE. Discard if vaccine has been frozen.
Between uses, return the multi-dose vial to the recommended storage conditions at 2° to 8°C (35° to 46°F).
Do not use after the expiration date shown on the label.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
- Inform the patient or guardian that Fluzone contains killed viruses and cannot cause influenza.
- Fluzone stimulates the immune system to produce antibodies that help protect against influenza, but does not prevent other respiratory infections.
- Annual influenza vaccination is recommended.
- Instruct vaccine recipients and guardians to report adverse reactions to their healthcare provider and/or to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967.
- Vaccine Information Statements must be provided to vaccine recipients or their guardians, as required by the National Childhood Vaccine Injury Act of 1986 prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
- SPL UNCLASSIFIED SECTION
-
Patient Information Sheet Fluzone®Influenza Vaccine
Please read this information sheet before getting Fluzone vaccine. This summary is not intended to take the place of talking with your healthcare provider. If you have questions or would like more information, please talk with your healthcare provider.
What is Fluzone vaccine?
Fluzone is a vaccine that helps protect against influenza illness (flu).
Fluzone vaccine is for people who are 6 months of age and older.
Vaccination with Fluzone vaccine may not protect all people who receive the vaccine.
Who should not get Fluzone vaccine?
You should not get Fluzone vaccine if you:
- ever had a severe allergic reaction to eggs or egg products.
- ever had a severe allergic reaction after getting any influenza vaccine.
- are younger than 6 months of age.
Tell your healthcare provider if you or your child have or have had:
- Guillain-Barré syndrome (severe muscle weakness) after getting an influenza vaccine.
- problems with your immune system as the immune response may be diminished.
How is the Fluzone vaccine given?
Fluzone vaccine is given as an injection into the muscle.
What are the possible side effects of Fluzone vaccine?
The most common side effects of Fluzone vaccine are:
- pain, redness, swelling, bruising and hardness where you got the injection
- muscle aches
- tiredness
- headache
- fever
These are not all of the possible side effects of Fluzone vaccine. Ask your healthcare provider about other side effects.
Call your healthcare provider for advice about any side effects that concern you. You may report side effects to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or http://vaers.hhs.gov.
What are the ingredients in Fluzone vaccine?
Fluzone vaccine contains 3 killed influenza virus strains.
Other ingredients include formaldehyde and octylphenol ethoxylate. The preservative thimerosal is only in the multi-dose vial of Fluzone vaccine.
Manufactured by:
Sanofi Pasteur Inc.
Swiftwater, PA 18370 USA -
PRINCIPAL DISPLAY PANEL - 5 mL Multi-Dose Vial
NDC: 49281-643-78
Influenza Vaccine
Fluzone®
2025-2026 Formula
Contains Preservative. DO NOT FREEZE. Store at 2° to 8°C (35° to 46°F).
SHAKE WELL. Contents: One 5 mL multi-dose vial
0.25 mL or 0.5 mL dose for 6 - 35 months.
0.5 mL dose for 3 years of age and older.
For Intramuscular Use. See package insert for details.
US Govt License #1725
Mfg by: Sanofi Pasteur Inc.934295
Lot XXXXXXX
EXP XXXX/XX
-
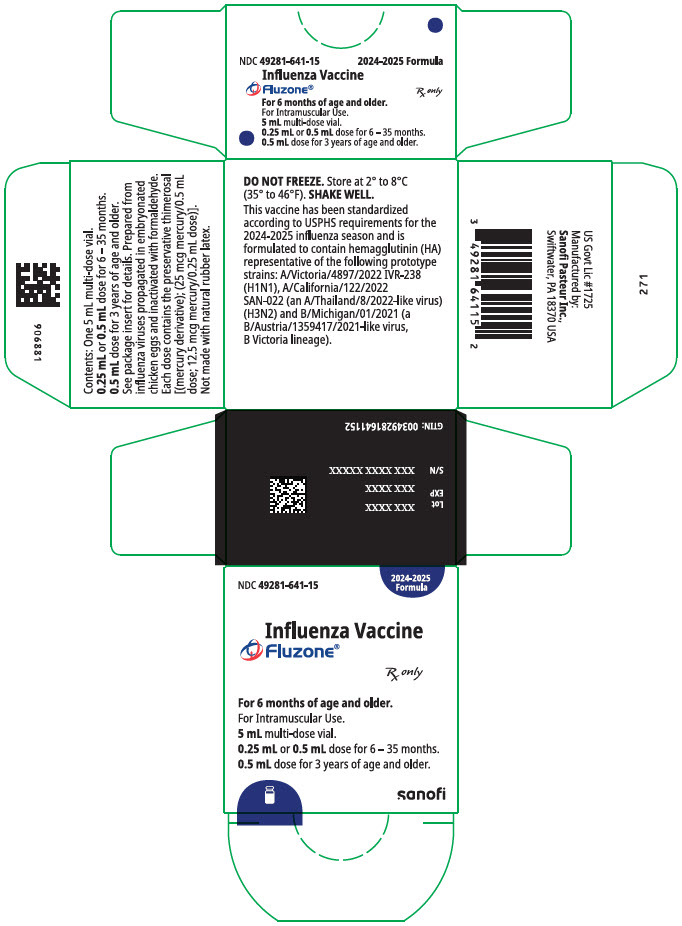
PRINCIPAL DISPLAY PANEL - 5 mL Vial Package
NDC: 49281-643-15
2025-2026
FormulaInfluenza Vaccine
Fluzone®Rx only
For 6 months of age and older.
For Intramuscular Use.
5 mL multi-dose vial.
0.25 mL or 0.5 mL dose for 6 - 35 months.
0.5 mL dose for 3 years of age and older.sanofi
-
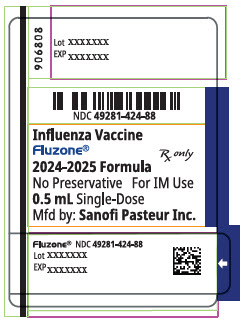
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
NDC: 49281-125-88
Influenza Vaccine
Fluzone®
Rx only2025-2026 Formula
No Preservative
For IM Use0.5 mL Single-Dose
Mfd by: Sanofi Pasteur Inc.
-
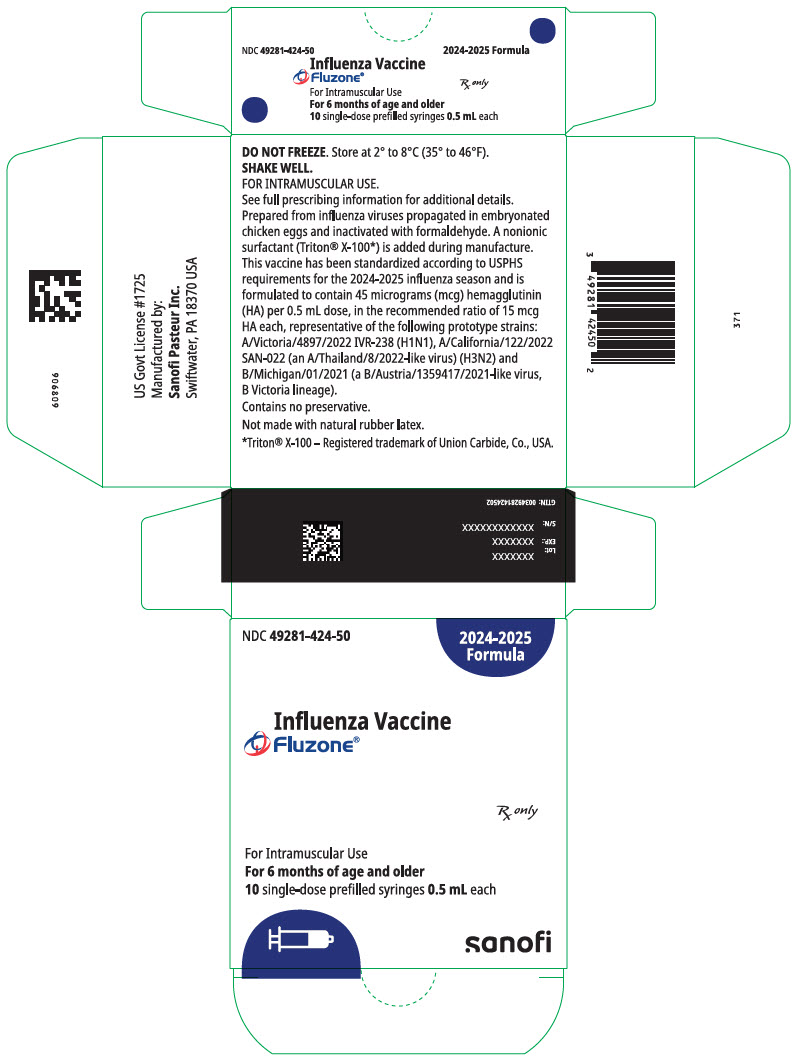
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Package
NDC: 49281-425-50
2025-2026
FormulaInfluenza Vaccine
Fluzone®Rx only
For Intramuscular Use
For 6 months of age and older
10 single-dose prefilled syringes 0.5 mL eachsanofi
-
INGREDIENTS AND APPEARANCE
FLUZONE TRIVALENT NORTHERN HEMISPHERE
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136/rv/2023 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-643 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: AU5C98U4BB) (INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:C46XJT9FQ9) INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: HQW9FZS4YK) (INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:SD82XNG5M6) INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/MICHIGAN/01/2021 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: FF9YP4D23C) (INFLUENZA B VIRUS B/MICHIGAN/01/2021 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:CQV855H5FG) INFLUENZA B VIRUS B/MICHIGAN/01/2021 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength OCTOXYNOL-9 (UNII: 7JPC6Y25QS) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) WATER (UNII: 059QF0KO0R) FORMALDEHYDE (UNII: 1HG84L3525) THIMEROSAL (UNII: 2225PI3MOV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-643-15 1 in 1 PACKAGE 1 NDC: 49281-643-78 5 mL in 1 VIAL, MULTI-DOSE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103914 07/01/2025 06/30/2026 FLUZONE TRIVALENT NORTHERN HEMISPHERE
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136/rv/2023 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-425 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: AU5C98U4BB) (INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:C46XJT9FQ9) INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: HQW9FZS4YK) (INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:SD82XNG5M6) INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/MICHIGAN/01/2021 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: FF9YP4D23C) (INFLUENZA B VIRUS B/MICHIGAN/01/2021 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:CQV855H5FG) INFLUENZA B VIRUS B/MICHIGAN/01/2021 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength OCTOXYNOL-9 (UNII: 7JPC6Y25QS) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) WATER (UNII: 059QF0KO0R) FORMALDEHYDE (UNII: 1HG84L3525) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-425-50 10 in 1 PACKAGE 1 NDC: 49281-425-88 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103914 07/01/2024 Labeler - Sanofi Pasteur Inc. (086723285) Registrant - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Inc. 086723285 MANUFACTURE(49281-425, 49281-643) , PACK(49281-425, 49281-643) , LABEL(49281-425, 49281-643)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.