SucroMate® Equine (deslorelin acetate)
SucroMate Equine by
Drug Labeling and Warnings
SucroMate Equine by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUCROMATE EQUINE- deslorelin acetate injection, suspension
Dechra Veterinary Products
----------
SucroMate® Equine
(deslorelin acetate)
Sterile Suspension
Injectable sustained release gonadotropin releasing hormone (GnRH) analog
For use in horses only
For intramuscular injection
Refrigerate after each use
DESCRIPTION:
SucroMate Equine (deslorelin acetate) is a sterile, synthetic gonadotropin-releasing hormone (GnRH) analog suspension. SucroMate Equine is a sustained release formulation that forms an in situ gel upon intramuscular injection. Deslorelin acetate is [(6-D-tryptophan-9-(N-ethyl-L-prolinamide)-10-deglycinamide)] GnRH. The molecular weight is 1282.6.
SucroMate Equine
The deslorelin acetate structural formula is:
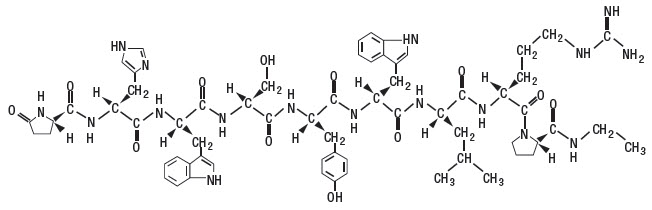
[(6-D-tryptophan-9-(N-ethyl-L-prolinamide)-10-deglycinamide)] GnRH
Each mL contains 1.8 mg deslorelin acetate (1.7 mg deslorelin) for injection as a sterile suspension in sucrose acetate isobutyrate/propylene carbonate (70:30 wt:wt). Ten doses per vial.
INDICATION:
SucroMate Equine is indicated for inducing ovulation within 48 hours of treatment in cyclic estrous mares with an ovarian follicle between 30 and 40 mm in diameter.
DOSAGE and ADMINISTRATION:
Shake well before use. Administer 1 mL (one dose) per estrus cycle, 48 hours prior to desired ovulation. Verify that the mare is in estrus and has at least one ovarian follicle between 30 and 40 mm in diameter.
SucroMate Equine is a suspension of deslorelin and settling will occur over time. Warm the product for 2 minutes by rolling the vial between the palms (to reach room temperature), or allow the vial to sit for 30 minutes at room temperature prior to administration. Shake the vial vigorously for 1 minute before use. Administer a 1 mL intramuscular injection into the thick musculature of the neck.
Prior to administration, it should be determined by rectal palpation and/or ultrasonography that the cyclic estrous mare has an ovarian follicle between 30 and 40 mm in diameter. Effectiveness is contingent upon accurate diagnosis of estrus and detection of a developing follicle between 30 and 40 mm in diameter. Only 1 mL should be administered per mare during a given estrus.
CONTRAINDICATIONS:
SucroMate Equine is contraindicated in horses known to be hypersensitive to deslorelin acetate.
WARNING:
For use in horses (estrous mares) only. Do not use in horses intended for human consumption.
For intramuscular (IM) use only. Do not administer intravascularly. Not for use in humans. Keep this and all drugs out of reach of children.
HUMAN WARNING – NOT FOR HUMAN USE
Pregnant women and women of childbearing age should exercise caution when handling this product. Accidental administration may lead to a disruption of the menstrual cycle. Direct contact with the skin should be avoided. If exposure occurs, contact areas should be washed immediately with alcohol followed by soap and water, as this product is insoluble in water. In case of accidental human injection, consult a physician immediately.
To obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
PRECAUTIONS:
The use of GnRH analogs in cycling mares has been associated with prolonged interovulatory intervals. SucroMate Equine has not been evaluated in mares less than 3 years of age.
ADVERSE REACTIONS:
Injection site swelling was observed following the administration of SucroMate Equine during the effectiveness and safety studies; all injection site swellings resolved within 5 days, and 7 – 14 days, respectively.
CONTACT INFORMATION:
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at http://www.fda.gov/reportanimalae
CLINICAL PHARMACOLOGY:
Deslorelin induces ovulation by increasing the levels of endogenous luteinizing hormone.
SucroMate Equine has been shown to induce ovulation within 48 hours of treatment in 77% of mares with a developing follicle between 30 and 40 mm in diameter. This product is intended to optimize breeding management through induction of ovulation within 48 hours.
EFFECTIVENESS:
Two hundred eighty three (283) mares, ranging in age from 2 to 20 years (mean 10.6 years) of age randomly received either SucroMate Equine or placebo after the presence of an ovarian follicle between 30 and 40 mm in diameter was confirmed. All 283 mares were evaluated for field safety under actual conditions of use. A total of 191 mares (97 placebo and 94 mares in the SucroMate Equine group) were evaluated for effectiveness over a total of 273 cycles (131 placebo and 142 SucroMate Equine cycles). Breeds included Standardbreds, Thoroughbreds, Quarter Horses and Paints. Mares were evaluated for up to 3 cycles to determine if SucroMate Equine induced ovulation in cycling mares, and to determine the effects on the interovulatory interval, pregnancy rate and general health characteristics of the resultant foals.
The proportion of mares ovulating within 48 hours of treatment was significantly higher in the SucroMate Equine group as compared to the placebo group (see Table 1).
| Placebo (1.0 mL) | SucroMate Equine (1.0 mL) | P value | |
|---|---|---|---|
|
|
|||
| 1st Cycle | |||
| Number of mares treated | 97 | 94 | |
| Yes | 23 (24%) | 68 (72%) | p = 0.000* |
| No | 73 (75%) | 25 (27%) | |
| Not determined | 1 (1%) | 1 (1%) | |
| 2nd Cycle | |||
| Number of mares treated | 25 | 35 | |
| Yes | 9 (36%) | 29 (83%) | p = 0.000* |
| No | 16 (64%) | 6 (17%) | |
| 3rd Cycle | |||
| Number of mares treated | 9 | 13 | |
| Yes | 3 (33%) | 12 (92%) | p = 0.006* |
| No | 6 (67%) | 0 (0%) | |
| Not determined | 0 (0%) | 1 (8%) | |
| Percent of mares ovulating within 48 hours after treatment in any cycle | 27% | 77% | |
Following treatment in each of three estrus cycles, the mean hours to ovulation was reduced in the SucroMate Equine treated group compared to the placebo group (See Table 2).
| Placebo (1.0 mL) | SucroMate Equine (1.0 mL) | P value | |
|---|---|---|---|
|
|
|||
| 1st Cycle | 91.8 (n=96) | 65.1 (n=90) | p = 0.019† |
| 2nd Cycle | 83.0 (n=24) | 56.2 (n=35) | p = 0.099 |
| 3rd Cycle | 85.3 (n=9) | 41.0 (n=12) | p = 0.153 |
The interovulatory interval was calculated between cycles for all mares that did not become pregnant in the previous cycle. There was no significant difference in the length of interovulatory intervals between the SucroMate Equine (deslorelin acetate) treated group and the placebo-treated mares as represented in Table 3.
| Placebo (1.0 mL) | SucroMate Equine (1.0 mL) | P value | |
|---|---|---|---|
|
|
|||
| Cycles 1-2 | 26.1 (n=27) | 22.3 (n=32) | p = 0.182 |
| Cycles 2-3 | 21.3 (n=9) | 18.5 (n=11) | p = 0.336 |
| Cycles 3-4 | 13.0 (n=1) | 18.0 (n=1) | |
Twins were detected in 8 placebo mares and 2 SucroMate Equine mares. The nursing behavior, mobility and general health of the live foals were similar for foals from mares in the SucroMate Equine and placebo groups. See Table 4 for a summary of the foaling and foal assessment data.
| Treatment Group | ||
|---|---|---|
| Assessment | Placebo | SucroMate Equine |
|
|
||
| Mares confirmed pregnant in 1999 breeding season out of total # of mares per group % (n/N) | 83% (118/143) | 81% (114/140) |
| Mares confirmed to have a live foal out of # of pregnant mares % (n/N) | 94% (111/118) | 84% (96/114) |
| Mares confirmed to have a stillbirth out of # of pregnant mares % (n/N) | 0% (0/118) | 3.0% (3/114) |
| 72 Hour-General Health Foal Assessment % (n/N) Good Satisfactory Not Satisfactory | 80% (89/111) 16% (18/111) 2% (2/111) | 79% (76/96) 20% (19/96) 2% (2/96) |
| 4 Week-General Health Foal Assessment % (n/N) Good Not Assessed | 34% (38/111) 66% (73/111) | 36% (35/96) 67% (64/96) |
When administered to cycling, estrous mares with a pre-ovulatory follicle between 30 mm and 40 mm in diameter, a single, 1 mL injection of SucroMate Equine (1.8 mg of deslorelin acetate) resulted in ovulation within 48 hours of treatment in 77% of treated mares. The length of the interovulatory interval between cycles was not altered.
The pregnancy rate of the treated mares was similar to the placebo mares.
Animal Safety: In a target animal safety study, 32 reproductively sound, mixed breed mares, ranging in age from 3-15 years were randomly assigned to one of four dose groups; each dose group contained 8 mares. The dose groups were administered 0X (placebo), 1X (1 mL), 3X (3 mL) or 5X (5 mL) the recommended 1.8 mg dose of SucroMate Equine for three consecutive cycles.
Treatment with SucroMate Equine at all doses reduced the mean hours to ovulation during all three treatment cycles compared to the placebo group. Of the sixty-five treatment experiences for the SucroMate Equine treated mares across the three cycles 55 (84.6%) were followed by ovulation within 48 hours compared to five of twenty-three (21.7%) for placebo mares. There was no statistically significant effect on the length of estrus at any dose level.
Some mares in all SucroMate Equine treatment groups exhibited mild to moderate swelling at the injection site six hours post-injection during all three treatment cycles. All injection site reactions in the 1X treated mares resolved by Day 3 post-treatment. Two mares, one 3X mare and one 5X mare, had swelling and hardness noted at injection sites at Day 7 post-treatment. The incidence of injection site reactions for the placebo, 1X, 3X and 5X groups was 37%, 75%, 50%, and 87%, respectively.
The LH concentrations in the SucroMate Equine treated groups in all three cycles exhibited an expected surge following treatment and prior to ovulation, followed by lower concentrations during the midluteal phase, with a gradual return to pretreatment levels.
The mean interovulatory interval in the SucroMate Equine groups ranged from 17.8 - 23 days compared to 21 - 23.5 days in the placebo group.
All mares that ovulated after the 3rd treatment with SucroMate Equine or placebo were bred.
These mares were bred until they became pregnant (not to exceed three cycles). In the placebo group, seven mares ovulated after the 3rd treatment and were bred. Six of the seven mares became pregnant (85.7%). In the 1X group, six mares ovulated after the 3rd treatment and were bred. All six became pregnant (100%). In the 3X group, eight mares ovulated after the 3rd treatment and were bred. Six of the eight mares became pregnant (75%). In the 5X group, five mares ovulated after the 3rd treatment and all became pregnant (100%).
There were no significant differences across the treatment groups in terms of time to pregnancy or the number and proportion of mares that became pregnant after three consecutive SucroMate Equine injections.
In a separate dose tolerance study mares received a single IM injection of placebo (8 mares) or SucroMate Equine at 10X the recommended 1.8 mg dose (8 mares). One SucroMate Equine 10X mare exhibited moderate tremors and hives at 6 hours post-treatment.
Approved by FDA under NADA # 141-319
Manufactured for:
Dechra Veterinary Products
7015 College Boulevard, Suite 525
Overland Park, KS 66211 USA
SucroMate is a registered trademark of Dechra Limited
Rev. August 2022
SP-1000595
Dechra
PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
SucroMate®
Equine
(deslorelin acetate)
Sterile Suspension
For use in horses only
For Intramuscular Injection
CAUTION: Federal law restricts
this drug to use by or on the
order of a licensed veterinarian.
A sustained release gonadotropin
releasing hormone (GnRH) analog
(deslorelin acetate) for ovulation
induction in estrous mares
Approved by FDA under
NADA # 141-319
Net Contents:
10 mL
Dechra

| SUCROMATE EQUINE
deslorelin acetate injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dechra Veterinary Products (362142734) |