ANTIVENIN- latrodectus mactans kit
ANTIVENIN by
Drug Labeling and Warnings
ANTIVENIN by is a Other medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Antivenin (Latrodectus mactans) is a sterile, non-pyrogenic preparation derived by drying a frozen solution of specific venom-neutralizing globulins obtained from the blood serum of healthy horses immunized against venom of black widow spiders (Latrodectus mactans). It is standardized by biological assay on mice, in terms of one dose of Antivenin neutralizing the venom in not less than 6000 mouse LD50 of Latrodectus mactans. Thimerosal (mercury derivative) 1:10,000 is added as a preservative. When constituted as specified, it is opalescent, ranging in color from light (straw) to very dark (iced tea), and contains not more than 20.0 percent of solids.
Each single-dose vial contains not less than 6000 Antivenin units. One unit of Antivenin will neutralize one average mouse lethal dose of black widow spider venom when the Antivenin and the venom are injected simultaneously in mice under suitable conditions.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Antivenin (Latrodectus mactans) is used to treat patients with symptoms due to bites by the black widow spider (Latrodectus mactans). Early use of the Antivenin is emphasized for prompt relief.
Local muscular cramps begin from 15 minutes to several hours after the bite which usually produces a sharp pain similar to that caused by puncture with a needle. The exact sequence of symptoms depends somewhat on the location of the bite. The venom acts on the myoneural junctions or on the nerve endings, causing an ascending motor paralysis or destruction of the peripheral nerve endings. The groups of muscles most frequently affected at first are those of the thigh, shoulder, and back. After a varying length of time, the pain becomes more severe, spreading to the abdomen, and weakness and tremor usually develop. The abdominal muscles assume a boardlike rigidity, but tenderness is slight. Respiration is thoracic. The patient is restless and anxious. Feeble pulse, cold, clammy skin, labored breathing and speech, light stupor, and delirium may occur. Convulsions also may occur, particularly in small children. The temperature may be normal or slightly elevated. Urinary retention, shock, cyanosis, nausea and vomiting, insomnia, and cold sweats also have been reported. The syndrome following the bite of the black widow spider may be confused easily with any medical or surgical condition with acute abdominal symptoms.
The symptoms of black widow spider bite increase in severity for several hours, perhaps a day, and then very slowly become less severe, gradually passing off in the course of two or three days except in fatal cases. Residual symptoms such as general weakness, tingling, nervousness, and transient muscle spasm may persist for weeks or months after recovery from the acute stage.
If possible, the patient should be hospitalized. Other additional measures giving greatest relief are prolonged warm baths and intravenous injection of 10 mL of 10 percent solution of calcium gluconate repeated as necessary to control muscle pain. Morphine also may be required to control pain. Barbiturates may be used for extreme restlessness. However, as the venom is a neurotoxin, it can cause respiratory paralysis. This must be borne in mind when considering use of morphine or a barbiturate. Adrenocorticosteroids have been used with varying degrees of success. Supportive therapy is indicated by the condition of the patient. Local treatment of the site of the bite is of no value. Nothing is gained by applying a tourniquet or by attempting to remove venom from the site of the bite by incision and suction.
In otherwise healthy individuals between the ages of 16 and 60, the use of Antivenin may be deferred and treatment with muscle relaxants may be considered.
-
WARNINGS
Prior to treatment with any product prepared from horse serum, a careful review of the patient's history should be taken emphasizing prior exposure to horse serum or any allergies. Serum sickness and even death could result from the use of horse serum in a sensitive patient. A skin or conjunctival test should be performed prior to administration of Antivenin. However, an anaphylactic reaction to Antivenin may occur even following a negative skin or conjunctival test (see ADVERSE REACTIONS).
Skin test: Inject into (not under) the skin not more than 0.02 mL of the test material (1:10 dilution of normal horse serum in physiologic saline). Evaluate result in 10 minutes. A positive reaction is an urticarial wheal surrounded by a zone of erythema. A control test using Sodium Chloride Injection facilitates interpretation of the results.
Conjunctival test: For adults instill into the conjunctival sac one drop of a 1:10 dilution of horse serum and for children one drop of 1:100 dilution. Itching of the eye and reddening of the conjunctiva indicate a positive reaction, usually within 10 minutes.
Patients should be observed for serum sickness for an average of 8 to 12 days following administration of Antivenin.
Desensitization should be attempted only when the administration of Antivenin is considered necessary to save life. Epinephrine must be available in case of untoward reaction.
Desensitization: If the history is positive or the results of the sensitivity tests are mildly or questionably positive, Antivenin should be administered as follows to reduce the risk of an immediate severe allergic reaction:
- In separate sterile vials or syringes prepare 1:10 or 1:100 dilutions of Antivenin in Sodium Chloride for Injection.
- Allow at least 15 but preferably 30 minutes between injections and only proceed with the next dose if no reactions occurred following the previous dose.
- Using a tuberculin syringe, inject subcutaneously 0.1, 0.2 and 0.5 mL of the 1:100 dilution at 15 or 30 minute intervals; repeat with the 1:10 dilution, and finally the undiluted Antivenin.
- If there is a reaction after any of the injections, place a tourniquet proximal to the sites of injection and administer epinephrine, 1:1000 (0.3 to 1.0 mL subcutaneously, 0.05 to 0.1 mL intravenously), proximal to the tourniquet or into another extremity. Wait at least 30 minutes before giving another injection of Antivenin, the amount of which should be the same as the last one not evoking a reaction.
- If no reaction has occurred after 0.5 mL of undiluted Antivenin has been given, it is probably safe to continue the dose at 15 minute intervals until the entire dose has been injected.
-
PRECAUTIONS
Patients with Asthma
Anaphylactic reactions and death have been reported in patients with a medical history of asthma.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term studies in animals have been performed to evaluate the potential for carcinogenesis, mutagenesis, or impairment of fertility.
Pregnancy
Animal reproduction studies have not been conducted with Black Widow Spider Antivenin. It is also not known whether Black Widow Spider Antivenin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Black Widow Spider Antivenin should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Black Widow Spider Antivenin is administered to a nursing woman.
Pediatric Use
Controlled clinical studies for safety and effectiveness in children have not been conducted.
Geriatric Use
Reported clinical experience has not identified differences in responses between the elderly and younger patients. Because of the increased risk of complications from envenomation in elderly patients, the standard of care described in the literature suggests that patients older than 60 years of age should be given Antivenin as a preferred initial therapy (see INDICATIONS AND USAGE).
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Using a sterile syringe, inject 2.5 mL of Sterile Water for Injection into the single-dose vial of Antivenin. With the needle still in the rubber stopper, shake the vial to dissolve the contents completely.
Parenteral drug products should be inspected visually for particulate matter prior to administration, whenever solution and container permit (see DESCRIPTION).
The dose for adults and children is the entire contents of a restored single-dose vial (2.5 mL) of Antivenin. It may be given intramuscularly, preferably in the region of the anterolateral thigh so that a tourniquet may be applied in the event of a systemic reaction. Symptoms usually subside in 1 to 3 hours. Although one dose of Antivenin usually is adequate, a second dose may be necessary in some cases. Discard unused portion.
Antivenin also may be given intravenously in 10 to 50 mL of saline solution over a 15 minute period. It is the preferred route in severe cases, or when the patient is under 12, or in shock. One restored single-dose vial usually is enough. Discard unused portion.
-
HOW SUPPLIED
No. 5424 — Antivenin (Latrodectus mactans), equine origin is a white to gray crystalline powder, each single-dose vial containing not less than 6000 Antivenin units. Thimerosal (mercury derivative) 1:10,000 is added as preservative, NDC 0006-5424-02. A single-dose 1 mL vial of normal horse serum (1:10 dilution) for sensitivity testing is also included. Thimerosal (mercury derivative) 1:10,000 is added as preservative.
-
REFERENCES
Barron, W. E.: Spider Bites, J. Med. Ass. Georgia 49: 511-512, Oct. 1960.
Micks, D. W.: Insects and Other Arthropods of Medical Importance in Texas, Tex. Rep. Biol. & Med. 18: 624-635, Winter 1960.
Prince, G. E.: Arachnidism in Children, J. Pediat. 49: 101-108, July 1956.
Russell, F. E.: Injuries by Venomous Animals in the United States, J. Amer. Med. Ass. 177: 903-907, Sept. 30, 1961.
Russell, F. E.: Muscle Relaxants in Black Widow Spider (Latrodectus mactans) Poisoning, Amer. J. Med. Sci. 243: 159-162, Feb. 1962.
Russell, F. E.: Venom Poisoning, Rational Drug Therap. 5: 5-6, Aug. 1971.
-
SPL UNCLASSIFIED SECTION
Distributed by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAFor patent information: www.merck.com/product/patent/home.html
Copyright © 2013-2020 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.Revised: 02/2020
uspi-mk9001-i-2002r023
-
This is a representative sample of the packaging. Please see How Supplied section for a complete list of available packaging.
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 0006-5424-02
1 Single-dose 2.5 mL Vial
ANTIVENIN
(LATRODECTUS MACTANS)
(BLACK WIDOW SPIDER ANTIVENIN)
Equine OriginRx only
For Intramuscular or Intravenous Use
Manufactured and Distributed by:
Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO.,INC.
Whitehouse Station, NJ 08889, USA
U.S. Govt. Lic. No. 0002 Canadian Lic. No. 2
Copyright © 2013 Merck Sharp & Dohme Corp.,
a subsidiary of Merck & Co., Inc.
All rights reserved.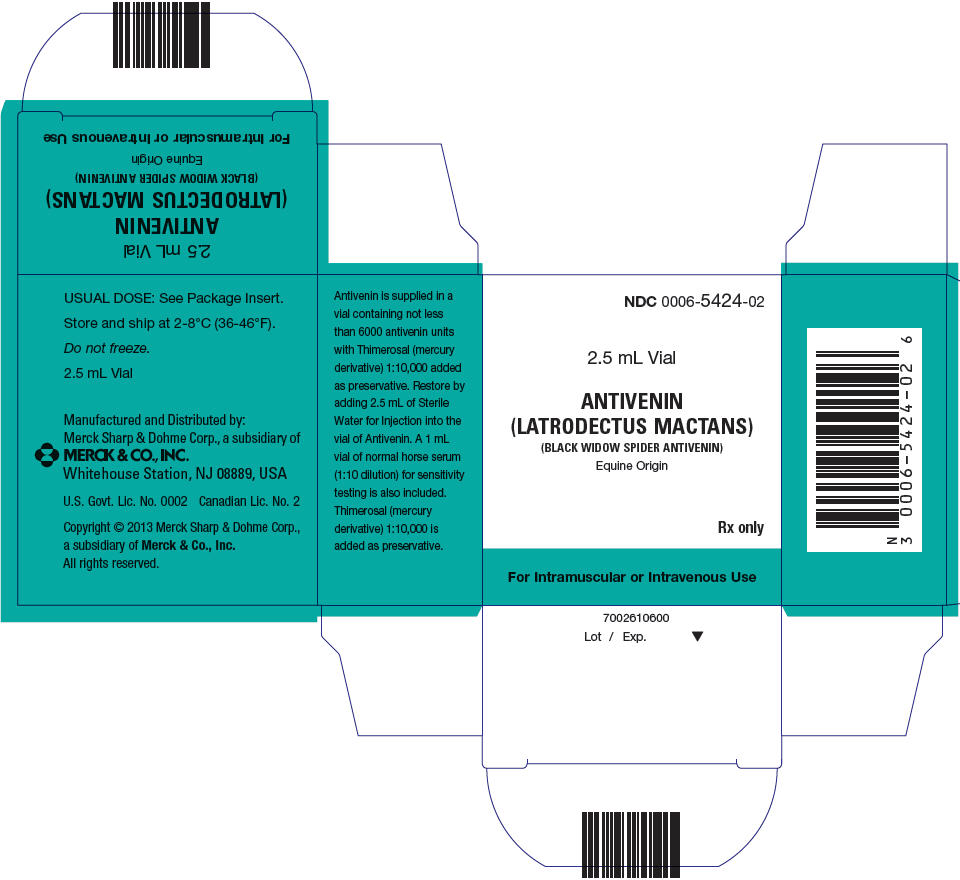
-
INGREDIENTS AND APPEARANCE
ANTIVENIN
latrodectus mactans kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0006-5424 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-5424-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 2.5 mL Part 2 1 VIAL 1 mL Part 1 of 2 ANTIVENIN
latrodectus mactans injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0006-4084 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK WIDOW SPIDER (LATRODECTUS MACTANS) IMMUNE GLOBULIN ANTIVENIN (EQUINE) (UNII: 5VJA14972G) (BLACK WIDOW SPIDER (LATRODECTUS MACTANS) IMMUNE GLOBULIN ANTIVENIN (EQUINE) - UNII:5VJA14972G) BLACK WIDOW SPIDER (LATRODECTUS MACTANS) IMMUNE GLOBULIN ANTIVENIN (EQUINE) 6000 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength THIMEROSAL (UNII: 2225PI3MOV) Product Characteristics Color BROWN (opalescent, ranges from light (straw) to very dark (iced tea)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-4084-01 2.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101062 05/14/1947 Part 2 of 2 NORMAL HORSE SERUM
normal horse serum solutionProduct Information Item Code (Source) NDC: 0006-9011 Route of Administration INTRADERMAL, CONJUNCTIVAL Inactive Ingredients Ingredient Name Strength SERUM, HORSE (UNII: QHD0E51VON) THIMEROSAL (UNII: 2225PI3MOV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-9011-00 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101084 01/02/1959 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101062 12/01/2014 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [ANTIVENIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ANTIVENIN 85105132 not registered Dead/Abandoned |
Boehringer Ingelheim Vetmedica, Inc. 2010-08-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.