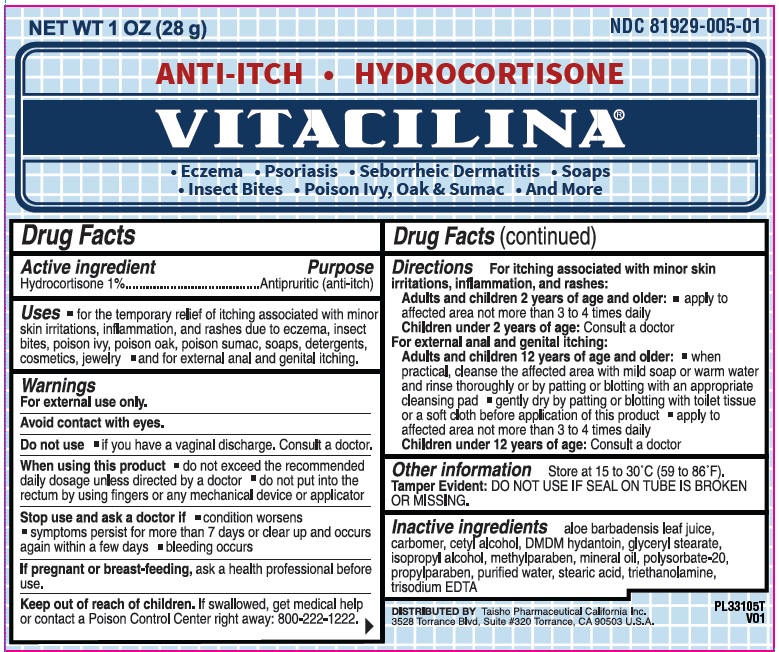
Vitacilina Anti Itch Hydrocortisone
Vitacilina Anti Itch Hydrocortisone by
Drug Labeling and Warnings
Vitacilina Anti Itch Hydrocortisone by is a Otc medication manufactured, distributed, or labeled by Taisho Pharmaceutical California Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VITACILINA ANTI ITCH HYDROCORTISONE- hydrocortisone cream
Taisho Pharmaceutical California Inc.
----------
Vitacilina Anti Itch Hydrocortisone
Uses
- for the temporary relief of itching associated with minor skin irritations, inflammation, and rashes due to eczema, insect bites, poison ivy, poison oak, poison sumac, soaps, detergents, cosmetics, jewelry
- and for external anal and genital itching.
Warnings
For external use only.
Avoid contact with eyes
When using this product
- do not exceed the recommended daily dosage unless directed by a doctor
- do not put into the rectum by using fingers or any mechanical device or applicator
Directions
- for itching of skin irritation, inflammation, and rashes:
Adults and children 2 years of age and older:
- apply to affected area not more than 3 to 4 times daily
Children under 2 years of age:consult a doctor
For external anal and genital itching:
Adults and children 12 years of age and older: - when practical, cleanse the affected area with mild soap or warm water and rinse thoroughly by patting or blotting with an appropriate cleansing pad
- gently dy by patting or blotting with toilet tissue or a soft cloth before appliction of this product
- apply to affected area not more than 3 to 4 times daily
- children under 12 years of age:consult a doctor
Other information
- store at 15 to 30°C (59 to 86°F)
- Tamper Evident:DO NOT USE IF SEAL ON TUBE IS BROKEN OR MISSING.
| VITACILINA ANTI ITCH HYDROCORTISONE
hydrocortisone cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Taisho Pharmaceutical California Inc. (603827635) |
Revised: 2/2025
Document Id: 2d7c78dd-0379-7c51-e063-6294a90abecb
Set id: 60fa0ed9-aa38-477c-babc-4dcd80dfa1ca
Version: 3
Effective Time: 20250206