VOL-PLUS- folic acid, ascorbic acid, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, niacinamide, vitamin a acetate, .beta.-carotene, cholecalciferol, .alpha.-tocopherol acetate, dl-, cupric oxide, zinc oxide, ferrous fumarate, and calcium sulfate tablet, coated
Vol-Plus by
Drug Labeling and Warnings
Vol-Plus by is a Other medication manufactured, distributed, or labeled by Trigen Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
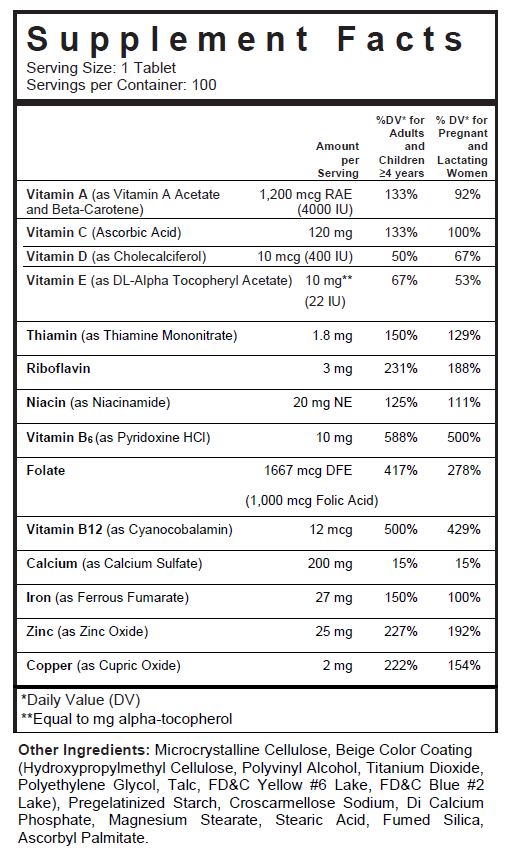
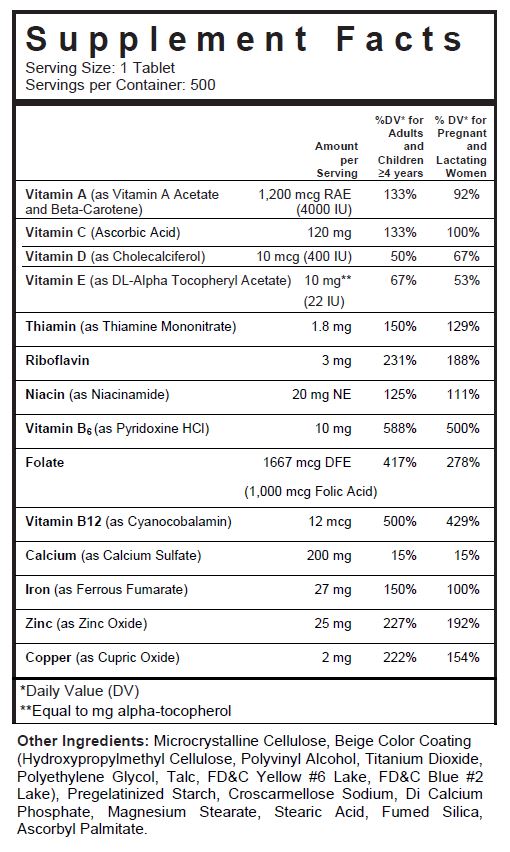
- SUPPLEMENT FACTS
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
-
DRUG INTERACTIONS
Pyridoxine hydrochloride should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine hydrochloride. However, pyridoxine hydrochloride may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
-
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid, as well as possibly the use of other forms of folates - including reduced folates.
Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride.
Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with cyanocobalamin.Ferrous Fumarate
Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and subside with continuation of therapy and physician encouragement. Although, the absorption of iron is best when taken between meals, occasional gastrointestinal disturbances may be controlled giving Vol-Plus shortly after meals.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
-
STORAGE
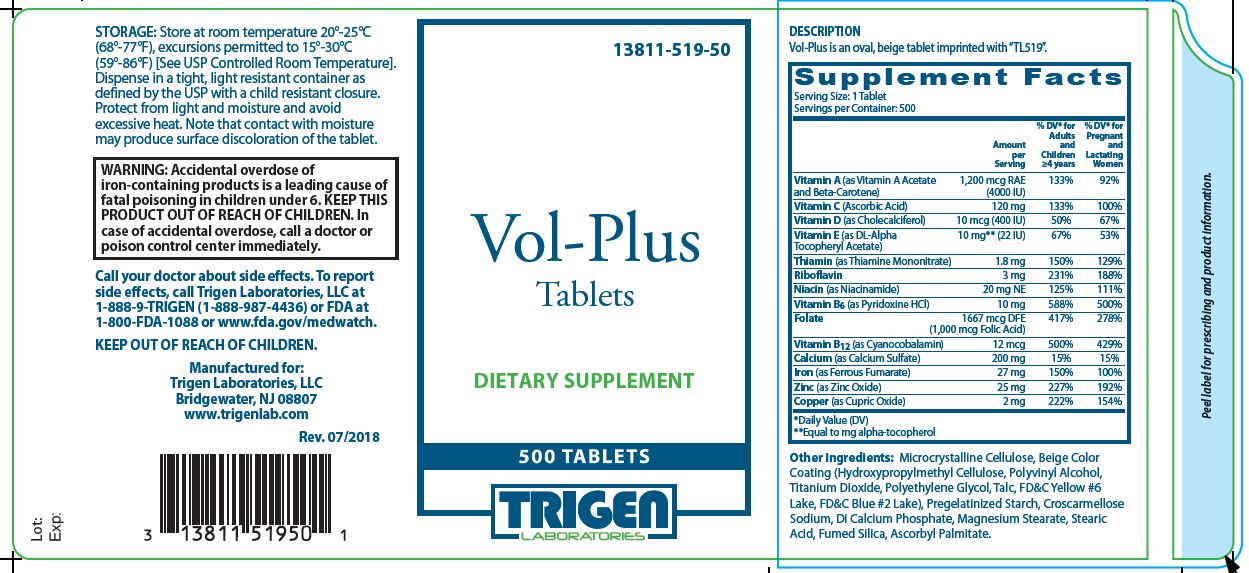
Store at room temperature 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°-86°F) [see USP controlled room temperature]. Dispense in a tight, light resistant container as defined by the USP with a child resistant closure. Protect from light and moisture and avoid excessive heat. Note that contact with moisture may produce surface discoloration of the tablet.
-
SAFE HANDLING WARNING
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-888-9-TRIGEN (1-888-987-4436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Rev. 07/2018Customer Service: 1-888-987-4436

Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807
www.trigenlab.com - PRINCIPAL DISPLAY PANEL - 500 Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL- 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
VOL-PLUS
folic acid, ascorbic acid, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, niacinamide, vitamin a acetate, .beta.-carotene, cholecalciferol, .alpha.-tocopherol acetate, dl-, cupric oxide, zinc oxide, ferrous fumarate, and calcium sulfate tablet, coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-519 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (Thiamine ION - UNII:4ABT0J945J) THIAMINE 1.84 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 500 [iU] .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 3500 [iU] CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 10 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg CALCIUM SULFATE (UNII: WAT0DDB505) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE 200 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) ASCORBYL PALMITATE (UNII: QN83US2B0N) HYPROMELLOSES (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-519-01 1000 in 1 BOTTLE, PLASTIC 2 NHRIC:13811-519-10 100 in 1 BOTTLE, PLASTIC 3 NHRIC:13811-519-50 500 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 06/01/2010 02/28/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 22 mm Labeler - Trigen Laboratories, LLC (830479668)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.