MUCINEX tablet, extended release
MUCINEX by
Drug Labeling and Warnings
MUCINEX by is a Otc medication manufactured, distributed, or labeled by DIRECT RX. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- OTC - WHEN USING SECTION
- INDICATIONS & USAGE SECTION
-
WARNINGS SECTION
Do not use
- for children under 12 years of age
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
-
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Do not use
for children under 12 years of ageAsk a doctor before use if you have
persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
cough accompanied by too much phlegm (mucus)Stop use and ask a doctor if
cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT SECTION
- OTC - QUESTIONS SECTION
- DOSAGE & ADMINISTRATION SECTION
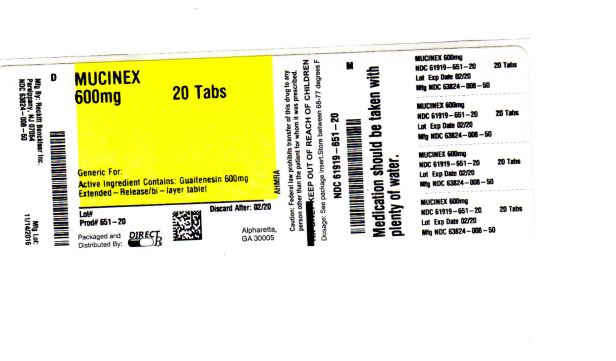
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MUCINEX
mucinex tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61919-651(NDC:63824-008) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape OVAL Size 16mm Flavor Imprint Code Mucinex;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61919-651-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021282 01/01/2014 Labeler - DIRECT RX (079254320) Establishment Name Address ID/FEI Business Operations DIRECT RX 079254320 relabel(61919-651) , repack(61919-651)
Trademark Results [MUCINEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCINEX 98499792 not registered Live/Pending |
RB Health (US) LLC 2024-04-15 |
 MUCINEX 90229382 not registered Live/Pending |
RB Health (US) LLC 2020-10-01 |
 MUCINEX 88040437 not registered Live/Pending |
Reckitt Benckiser LLC 2018-07-17 |
 MUCINEX 86191608 4613098 Live/Registered |
RB HEALTH (US) LLC 2014-02-12 |
 MUCINEX 85496330 4168070 Live/Registered |
RB HEALTH (US) LLC 2011-12-15 |
 MUCINEX 85489681 4168052 Live/Registered |
RB HEALTH (US) LLC 2011-12-07 |
 MUCINEX 77770135 3722363 Live/Registered |
Reckitt Benckiser Inc. 2009-06-29 |
 MUCINEX 76297961 2670161 Live/Registered |
RB HEALTH (US) LLC 2001-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.