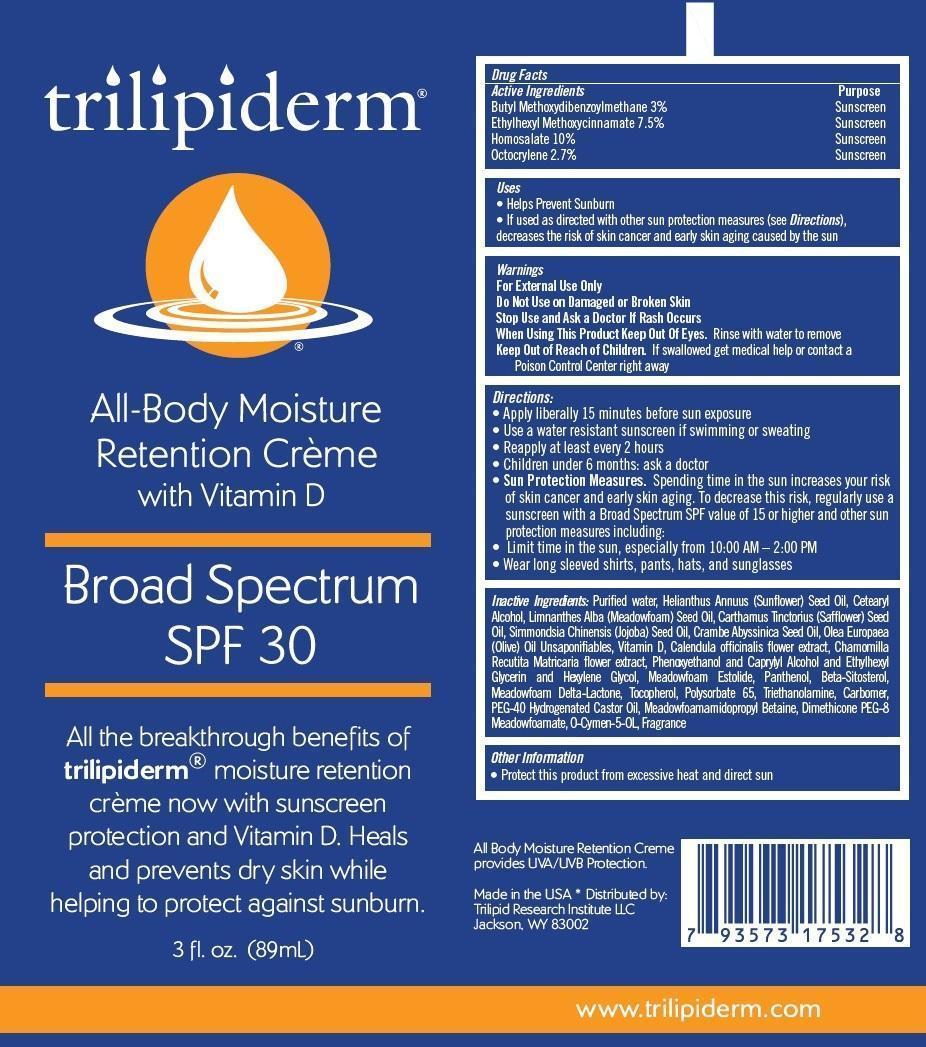
TRILIPIDERM BROAD SPECTRUM SPF 30- butyl methoxydibenzoylmethane, ethylhexyl methoxycinnamate, homosalate, octocrylene cream
TriLipiderm by
Drug Labeling and Warnings
TriLipiderm by is a Otc medication manufactured, distributed, or labeled by TriLipid Research Institute, LLC, Paket Corporation, RNA PHARMA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do Not Use
- Stop Use and Ask a Doctor
- When Using This Product
- Keep Out of Reach of Children.
-
Directions:
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10:00 AM – 2:00 PM
- Wear long sleeved shirts, pants, hats, and sunglasses
-
Inactive Ingredients:
Purified water, Helianthus Annuus (Sunflower) Seed Oil, Cetearyl Alcohol, Limnanthes Alba (Meadowfoam) Seed Oil, Carthamus Tinctorius (Safflower) Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Crambe Abyssinica Seed Oil, Olea Europaea (Olive) Oil Unsaponifiables, Vitamin D, Calendula officinalis flower extract, Chamomilla Recutita Matricaria flower extract, Phenoxyethanol and Caprylyl Alcohol and Ethylhexyl Glycerin and Hexylene Glycol, Meadowfoam Estolide, Panthenol, Beta-Sitosterol, Meadowfoam Delta-Lactone, Tocopherol, Polysorbate 65, Triethanolamine, Carbomer, PEG-40 Hydrogenated Castor Oil, Meadowfoamamidopropyl Betaine, Dimethicone PEG-8 Meadowfoamate, O-Cymen-5-OL, Fragrance
- Other Information
- SPL UNCLASSIFIED SECTION
- Trilipiderm Broad Spectrum SPF 30 Labels 64oz, 8oz, 3oz, 0.3oz, 0.14oz
-
INGREDIENTS AND APPEARANCE
TRILIPIDERM BROAD SPECTRUM SPF 30
butyl methoxydibenzoylmethane, ethylhexyl methoxycinnamate, homosalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60892-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.7 g in 100 mL Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PANTHENOL (UNII: WV9CM0O67Z) SODIUM BETA-SITOSTERYL SULFATE (UNII: I289C8TPSV) TOCOPHEROL (UNII: R0ZB2556P8) POLYSORBATE 65 (UNII: 14BGY2Y3MJ) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) PEG-40 CASTOR OIL (UNII: 4ERD2076EF) O-CYMEN-5-OL (UNII: H41B6Q1I9L) WATER (UNII: 059QF0KO0R) SUNFLOWER OIL (UNII: 3W1JG795YI) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) SAFFLOWER OIL (UNII: 65UEH262IS) JOJOBA OIL (UNII: 724GKU717M) CRAMBE HISPANICA SUBSP. ABYSSINICA SEED OIL (UNII: 0QW9S92J3K) OLIVE OIL (UNII: 6UYK2W1W1E) VITAMIN D (UNII: 9VU1KI44GP) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLIC ALCOHOL (UNII: NV1779205D) MEADOWLACTONE (UNII: 3OY5Q13U9R) MEADOWFOAMAMIDOPROPYL BETAINE (UNII: HNV0L650LG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60892-601-64 1896 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/31/2014 2 NDC: 60892-601-32 948 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/31/2014 3 NDC: 60892-601-04 1 in 1 BOX 01/31/2014 3 NDC: 60892-601-08 237 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC: 60892-601-05 1 in 1 BOX 12/13/2018 4 NDC: 60892-601-03 89 mL in 1 TUBE; Type 0: Not a Combination Product 5 NDC: 60892-601-01 29.625 mL in 1 TUBE; Type 0: Not a Combination Product 01/31/2014 6 NDC: 60892-601-00 9 mL in 1 PACKET; Type 0: Not a Combination Product 01/31/2014 7 NDC: 60892-601-77 4 mL in 1 PACKET; Type 0: Not a Combination Product 12/12/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/31/2014 Labeler - TriLipid Research Institute, LLC (013903326) Establishment Name Address ID/FEI Business Operations Paket Corporation 007774730 pack(60892-601) Establishment Name Address ID/FEI Business Operations RNA PHARMA, LLC 079103999 manufacture(60892-601)
Trademark Results [TriLipiderm]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRILIPIDERM 85979700 4394078 Live/Registered |
Fanning, Francis G. 2010-08-02 |
 TRILIPIDERM 85975589 4036447 Live/Registered |
Fanning, Francis G. 2010-08-02 |
 TRILIPIDERM 85097758 not registered Dead/Abandoned |
Fanning, Francis G. 2010-08-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 8oz label
8oz label
 3oz label
3oz label
 0.3oz label
0.3oz label


 3oz. Label
3oz. Label
 3 oz Box
3 oz Box
