JUXTAPID- lomitapide mesylate capsule
Juxtapid by
Drug Labeling and Warnings
Juxtapid by is a Prescription medication manufactured, distributed, or labeled by Chiesi USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use JUXTAPID safely and effectively. See full prescribing information for JUXTAPID.
JUXTAPID® (lomitapide) capsules, for oral use
Initial U.S. Approval: 2012WARNING: RISK OF HEPATOTOXICITY
See full prescribing information for complete boxed warning.
JUXTAPID can cause elevations in transaminases (5.1).
- Measure alanine and aspartate aminotransferases (ALT, AST), alkaline phosphatase, and total bilirubin before initiating treatment and then ALT and AST regularly as recommended (2.4, 5.1).
- During treatment, adjust the dose of JUXTAPID if the ALT or AST is ≥3 times the upper limit of normal (ULN) (2.4, 5.1).
- Discontinue JUXTAPID for clinically significant liver toxicity (2.4, 5.1).
JUXTAPID increases hepatic fat (hepatic steatosis) with or without concomitant increases in transaminases (5.1).
- Hepatic steatosis associated with JUXTAPID may be a risk factor for progressive liver disease, including steatohepatitis and cirrhosis (5.1).
Because of the risk of hepatotoxicity, JUXTAPID is available only through a restricted program called the JUXTAPID REMS Program (5.2). Prescribe JUXTAPID only to patients with a clinical or laboratory diagnosis consistent with HoFH. The safety and effectiveness of JUXTAPID have not been established in patients with hypercholesterolemia who do not have HoFH (1).
INDICATIONS AND USAGE
JUXTAPID is a microsomal triglyceride transfer protein inhibitor indicated as an adjunct to a low-fat diet and other lipid-lowering treatments, including LDL apheresis where available, to reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein B (apo B), and non-high-density lipoprotein cholesterol (non-HDL-C) in patients with homozygous familial hypercholesterolemia (HoFH) (1).
Limitations of Use
DOSAGE AND ADMINISTRATION
- Before treatment, measure ALT, AST, alkaline phosphatase, and total bilirubin; obtain a negative pregnancy test in females of reproductive potential; and initiate a low-fat diet supplying <20% of energy from fat (2.1).
- Initiate treatment at 5 mg once daily. Titrate dose based on acceptable safety/tolerability: increase to 10 mg daily after at least 2 weeks; and then, at a minimum of 4-week intervals, to 20 mg, 40 mg, and up to the maximum recommended dose of 60 mg daily (2.1).
- Due to reduced absorption of fat-soluble vitamins/fatty acids: Take daily vitamin E, linoleic acid, alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) supplements (2.1, 5.4).
- Take once daily, whole, with water and without food, at least 2 hours after evening meal (2.2).
- Patients with end-stage renal disease on dialysis or with baseline mild hepatic impairment should not exceed 40 mg daily (2.5, 2.6).
DOSAGE FORMS AND STRENGTHS
Capsules: 5 mg, 10 mg, 20 mg, and 30 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. Discontinue JUXTAPID if pregnancy detected (5.3, 8.1, 8.3).
- Gastrointestinal adverse reactions occur in 93% of patients and could affect absorption of concomitant oral medications (5.5).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥28%) are diarrhea, nausea, vomiting, dyspepsia, and abdominal pain (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP3A4 inhibitors increase exposure to lomitapide. Strong and moderate CYP3A4 inhibitors are contraindicated with JUXTAPID. Patients must avoid grapefruit juice (4, 5.6, 7.1).
- When administered with weak CYP3A4 inhibitors, the dose of JUXTAPID should be decreased by half. The dosage of JUXTAPID may then be up-titrated to a maximum recommended dosage of 30 mg daily (2.3, 5.6, 7.2).
- Warfarin: Lomitapide increases plasma concentrations of warfarin. Monitor international normalized ratio (INR) regularly, especially with JUXTAPID dose adjustment (5.8, 7.3).
- Simvastatin and lovastatin exposure increase with JUXTAPID. Limit dose when co-administered with JUXTAPID due to myopathy risk (5.7, 7.4).
- P-glycoprotein (P-gp) Substrates: Consider dose reduction of P-gp substrate because of possible increased absorption with JUXTAPID (7.5).
- Bile Acid Sequestrants: Separate JUXTAPID dosing by at least 4 hours (7.6).
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF HEPATOTOXICITY
1 INDICATIONS AND USAGE
1.1 Homozygous Familial Hypercholesterolemia
2 DOSAGE AND ADMINISTRATION
2.1 Initiation and Maintenance of Therapy
2.2 Administration
2.3 Dosing with Cytochrome P450 3A4 Inhibitors
2.4 Dose Modification Based on Elevated Transaminases
2.5 Dosing in Patients with Renal Impairment
2.6 Dosing in Patients with Baseline Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hepatotoxicity
5.2 JUXTAPID REMS Program
5.3 Embryo-Fetal Toxicity
5.4 Reduced Absorption of Fat-Soluble Vitamins and Serum Fatty Acids
5.5 Gastrointestinal Adverse Reactions
5.6 Concomitant Use of CYP3A4 Inhibitors
5.7 Risk of Myopathy with Concomitant Use of Simvastatin or Lovastatin
5.8 Risk of Supratherapeutic or Subtherapeutic Anticoagulation with Warfarin
5.9 Risk of Malabsorption with Rare Hereditary Disorders of Galactose Intolerance
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Moderate and Strong CYP3A4 Inhibitors
7.2 Weak CYP3A4 Inhibitors
7.3 Warfarin
7.4 Simvastatin and Lovastatin
7.5 P-glycoprotein Substrates
7.6 Bile Acid Sequestrants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED / STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF HEPATOTOXICITY
JUXTAPID can cause elevations in transaminases. In the JUXTAPID clinical trial, 10 (34%) of the 29 patients treated with JUXTAPID had at least one elevation in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) ≥3× upper limit of normal (ULN). There were no concomitant clinically meaningful elevations of total bilirubin, international normalized ratio (INR), or alkaline phosphatase [see Warnings and Precautions (5.1)].
JUXTAPID also increases hepatic fat, with or without concomitant increases in transaminases. The median absolute increase in hepatic fat was 6% after both 26 and 78 weeks of treatment, from 1% at baseline, measured by magnetic resonance spectroscopy. Hepatic steatosis associated with JUXTAPID treatment may be a risk factor for progressive liver disease, including steatohepatitis and cirrhosis [see Warnings and Precautions (5.1)].
Measure ALT, AST, alkaline phosphatase, and total bilirubin before initiating treatment and then ALT and AST regularly as recommended. During treatment, adjust the dose of JUXTAPID if the ALT or AST are ≥3× ULN. Discontinue JUXTAPID for clinically significant liver toxicity [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
Because of the risk of hepatotoxicity, JUXTAPID is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the JUXTAPID REMS Program [see Warnings and Precautions (5.2)]. Prescribe JUXTAPID only to patients with a clinical or laboratory diagnosis consistent with HoFH. The safety and effectiveness of JUXTAPID have not been established in patients with hypercholesterolemia who do not have HoFH [see Indications and Usage (1)].
-
1 INDICATIONS AND USAGE
1.1 Homozygous Familial Hypercholesterolemia
JUXTAPID is indicated as an adjunct to a low-fat diet and other lipid-lowering treatments, including LDL apheresis where available, to reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein B (apo B), and non-high-density lipoprotein cholesterol (non-HDL-C) in patients with homozygous familial hypercholesterolemia (HoFH).
Limitations of Use
- The safety and effectiveness of JUXTAPID have not been established in patients with hypercholesterolemia who do not have HoFH, including those with heterozygous familial hypercholesterolemia (HeFH).
- The effect of JUXTAPID on cardiovascular morbidity and mortality has not been determined.
-
2 DOSAGE AND ADMINISTRATION
2.1 Initiation and Maintenance of Therapy
Before beginning treatment with JUXTAPID:
- Measure transaminases (ALT, AST), alkaline phosphatase, and total bilirubin [see Warnings and Precautions (5.1)];
- Obtain a negative pregnancy test in females of reproductive potential prior to initiating treatment with JUXTAPID [see Contraindications (4), Warnings and Precautions (5.3), Use in Specific Populations (8.1, 8.3)];
- Initiate a low-fat diet supplying <20% of energy from fat [see Warnings and Precautions (5.5)].
The recommended starting dosage of JUXTAPID is 5 mg once daily, and the dose should be escalated gradually based on acceptable safety and tolerability. Transaminases should be measured prior to any increase in dose [see Warnings and Precautions (5.1)]. The maintenance dosage of JUXTAPID should be individualized, taking into account patient characteristics such as goal of therapy and response to treatment, to a maximum of 60 mg daily as described in Table 1. Modify dosing for patients taking concomitant weak CYP3A4 inhibitors and for those with renal impairment or baseline hepatic impairment [see Dosage and Administration (2.3), (2.5), and (2.6)]. Monitor transaminases during treatment with JUXTAPID as described in Warnings and Precautions (5.1), and reduce or withhold dosing for patients who develop transaminase values ≥3× the upper limit of normal (ULN) [see Dosage and Administration (2.4)].
Table 1: Recommended Regimen for Titrating Dosage DOSAGE DURATION OF ADMINISTRATION BEFORE CONSIDERING INCREASE TO NEXT DOSAGE 5 mg daily At least 2 weeks 10 mg daily At least 4 weeks 20 mg daily At least 4 weeks 40 mg daily At least 4 weeks 60 mg daily Maximum recommended dosage To reduce the risk of developing a fat-soluble nutrient deficiency due to JUXTAPID's mechanism of action in the small intestine, patients treated with JUXTAPID should take daily supplements that contain 400 international units vitamin E and at least 200 mg linoleic acid, 210 mg alpha-linolenic acid (ALA), 110 mg eicosapentaenoic acid (EPA), and 80 mg docosahexaenoic acid (DHA) [see Warnings and Precautions (5.4)].
2.2 Administration
JUXTAPID should be taken once daily with a glass of water, without food, at least 2 hours after the evening meal because administration with food may increase the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.5)]. Patients should swallow JUXTAPID capsules whole. Capsules should not be opened, crushed, dissolved, or chewed.
2.3 Dosing with Cytochrome P450 3A4 Inhibitors
JUXTAPID is contraindicated with concomitant use of moderate and strong cytochrome P450 3A4 (CYP3A4) inhibitors [see Contraindications (4) and Drug Interactions (7.1)].
The recommended maximum dosage of JUXTAPID is 30 mg daily with concomitant use of weak CYP3A4 inhibitors (such as alprazolam, amiodarone, amlodipine, atorvastatin, bicalutamide, cilostazol, cimetidine, cyclosporine, fluoxetine, fluvoxamine, ginkgo, goldenseal, isoniazid, lapatinib, nilotinib, pazopanib, ranitidine, ranolazine, ticagrelor, zileuton). However, the recommended maximum dosage of JUXTAPID is 40 mg daily with concomitant use of oral contraceptives.
When initiating a weak CYP3A4 inhibitor in a patient already taking JUXTAPID 10 mg daily or more, decrease the dose of JUXTAPID by half; patients taking JUXTAPID 5 mg daily may continue with the same dosage. Careful titration of JUXTAPID may then be considered according to LDL-C response and safety/tolerability to a maximum recommended dosage of 30 mg daily except when coadministered with oral contraceptives, in which case the maximum recommended lomitapide dosage is 40 mg daily [see Drug Interactions (7.2)].
2.4 Dose Modification Based on Elevated Transaminases
Table 2 summarizes recommendations for dose adjustment and monitoring for patients who develop elevated transaminases during therapy with JUXTAPID [see Warnings and Precautions (5.1)].
Table 2: Dose Adjustment and Monitoring for Patients with Elevated Transaminases ALT OR AST TREATMENT AND MONITORING RECOMMENDATIONS* - * Recommendations based on an ULN of approximately 30-40 international units/L.
≥3× and <5× ULN - Confirm elevation with a repeat measurement within one week.
- If confirmed, reduce the dose and obtain additional liver-related tests if not
already measured (such as alkaline phosphatase, total bilirubin, and INR). - Repeat tests weekly and withhold dosing if there are signs of abnormal liver
function (increase in bilirubin or INR), if transaminase levels rise above 5× ULN,
or if transaminase levels do not fall below 3× ULN within approximately 4 weeks.
In these cases of persistent or worsening abnormalities, also investigate to identify
the probable cause. - If resuming JUXTAPID after transaminases resolve to <3× ULN, consider
reducing the dose and monitor liver-related tests more frequently.
≥5× ULN - Withhold dosing, obtain additional liver-related tests if not already measured
(such as alkaline phosphatase, total bilirubin, and INR), and investigate to identify
the probable cause. - If resuming JUXTAPID after transaminases resolve to <3× ULN, reduce the dose
and monitor liver-related tests more frequently.
If transaminase elevations are accompanied by clinical symptoms of liver injury (such as nausea, vomiting, abdominal pain, fever, jaundice, lethargy, flu-like symptoms), increases in bilirubin ≥2× ULN, or active liver disease, discontinue treatment with JUXTAPID and investigate to identify the probable cause [see Warnings and Precautions (5.1)].
2.5 Dosing in Patients with Renal Impairment
Patients with end-stage renal disease receiving dialysis should not exceed 40 mg daily. There are no data available to guide dosing in other patients with renal impairment [see Use in Specific Populations (8.6)].
2.6 Dosing in Patients with Baseline Hepatic Impairment
Patients with mild hepatic impairment (Child-Pugh A) should not exceed 40 mg daily [see Use in Specific Populations (8.7)].
-
3 DOSAGE FORMS AND STRENGTHS
5 mg: Orange/orange hard gelatin capsule printed with black ink "A733" and "5 mg"
10 mg: Orange/white hard gelatin capsule printed with black ink "A733" and "10 mg"
20 mg: White/white hard gelatin capsule printed with black ink "A733" and "20 mg"
30 mg: Orange/yellow hard gelatin capsule printed with black ink "A733" and "30 mg"
-
4 CONTRAINDICATIONS
JUXTAPID is contraindicated in the following conditions:
- Pregnancy [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1)].
- Concomitant administration of JUXTAPID with moderate or strong CYP3A4 inhibitors, as this can increase JUXTAPID exposure [see Warnings and Precautions (5.6), Drug Interactions (7.1), and Clinical Pharmacology (12.3)].
- Patients with moderate or severe hepatic impairment (based on Child-Pugh category B or C) and patients with active liver disease, including unexplained persistent elevations of serum transaminases [see Warnings and Precautions (5.1) and Use in Specific Populations (8.7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hepatotoxicity
JUXTAPID can cause elevations in transaminases and hepatic steatosis, as described below [see Warnings and Precautions (5.2)]. To what extent JUXTAPID-associated hepatic steatosis promotes the elevations in transaminases is unknown. Although cases of hepatic dysfunction (elevated transaminases with increase in bilirubin or INR) or hepatic failure have not been reported, there is concern that JUXTAPID could induce steatohepatitis, which can progress to cirrhosis over several years. The clinical studies supporting the safety and efficacy of JUXTAPID in HoFH would have been unlikely to detect this adverse outcome given their size and duration [see Clinical Studies (14)].
Elevation of Transaminases
Elevations in transaminases (alanine aminotransferase [ALT] and/or aspartate aminotransferase [AST]) are associated with JUXTAPID. In the clinical trial, 10 (34%) of the 29 patients with HoFH had at least one elevation in ALT or AST ≥3× ULN, and 4 (14%) of the patients had at least one elevation in ALT or AST ≥5× ULN. There were no concomitant or subsequent clinically meaningful elevations in bilirubin, INR, or alkaline phosphatase [see Adverse Reactions (6.1)].
During the 78-week HoFH clinical trial, no patients discontinued prematurely because of elevated transaminases. Among the 19 patients who subsequently enrolled in the HoFH extension study, one discontinued because of increased transaminases that persisted despite several dose reductions, and one temporarily discontinued because of markedly elevated transaminases (ALT 24× ULN, AST 13× ULN) that had several possible causes, including a drug-drug interaction between JUXTAPID and the strong CYP3A4 inhibitor clarithromycin [see Drug Interactions (7.1)].
Monitoring of Transaminases
Before initiating JUXTAPID and during treatment, monitor transaminases as recommended in Table 3.
Table 3: Recommendations for Monitoring Transaminases TIME RECOMMENDATIONS Before initiating treatment - Measure ALT, AST, alkaline phosphatase, and total bilirubin.
- If abnormal, consider initiating JUXTAPID only after an appropriate work-up and the baseline abnormalities have been explained or resolved.
- JUXTAPID is contraindicated in patients with moderate or severe hepatic impairment, or active liver disease, including unexplained persistent elevations of serum transaminases [see Contraindications (4)].
During the first year - Measure liver-related tests (ALT and AST, at a minimum) prior to each increase in dose or monthly, whichever occurs first.
After the first year - Measure liver-related tests (ALT and AST, at a minimum) at least every 3 months and before any increase in dose.
At any time during treatment - If transaminases are abnormal, reduce or withhold dosing of JUXTAPID and monitor as recommended [see Dosage and Administration (2.4)].
- Discontinue JUXTAPID for persistent or clinically significant elevations.
- If transaminase elevations are accompanied by clinical symptoms of liver injury (such as nausea, vomiting, abdominal pain, fever, jaundice, lethargy, flu-like symptoms), increases in bilirubin ≥2× ULN, or active liver disease, discontinue treatment with JUXTAPID and identify the probable cause.
Hepatic Steatosis
JUXTAPID increases hepatic fat, with or without concomitant increases in transaminases. Hepatic steatosis is a risk factor for progressive liver disease, including steatohepatitis and cirrhosis. The long-term consequences of hepatic steatosis associated with JUXTAPID treatment are unknown. During the HoFH clinical trial, the median absolute increase in hepatic fat was 6% after both 26 weeks and 78 weeks of treatment, from 1% at baseline, measured by magnetic resonance spectroscopy (MRS) [see Adverse Reactions (6.1)]. Clinical data suggest that hepatic fat accumulation is reversible after stopping treatment with JUXTAPID, but whether histological sequelae remain is unknown, especially after long-term use; protocol liver biopsies were not performed in the HoFH clinical trial.
Alcohol may increase levels of hepatic fat and induce or exacerbate liver injury. It is recommended that patients taking JUXTAPID should not consume more than one alcoholic drink per day.
Caution should be exercised when JUXTAPID is used with other medications known to have potential for hepatotoxicity, such as isotretinoin, amiodarone, acetaminophen (>4 g/day for ≥3 days/week), methotrexate, tetracyclines, and tamoxifen. The effect of concomitant administration of JUXTAPID with other hepatotoxic medications is unknown. More frequent monitoring of liver-related tests may be warranted.
JUXTAPID has not been studied concomitantly with other LDL-lowering agents that can also increase hepatic fat. Therefore, the combined use of such agents is not recommended.
5.2 JUXTAPID REMS Program
Because of the risk of hepatotoxicity associated with JUXTAPID therapy, JUXTAPID is available through a restricted program under the REMS. Under the JUXTAPID REMS, only certified healthcare providers and pharmacies may prescribe and distribute JUXTAPID. Further information is available at www.JUXTAPIDREMSProgram.com or by telephone at 1-85-JUXTAPID (1-855-898-2743).
5.3 Embryo-Fetal Toxicity
Based on findings from animal studies, JUXTAPID use is contraindicated in pregnancy since it may cause fetal harm [see Contraindications (4), Use in Specific Populations (8.1, 8.3)]. In animal reproduction studies in rats and ferrets, embryonic death and fetal malformations were observed at clinically relevant exposures. Females of reproductive potential should have a negative pregnancy test before starting JUXTAPID. Advise females of reproductive potential to use effective contraception during therapy with JUXTAPID and for two weeks after the final dose. If pregnancy is detected, discontinue JUXTAPID.
5.4 Reduced Absorption of Fat-Soluble Vitamins and Serum Fatty Acids
Given its mechanism of action in the small intestine, JUXTAPID may reduce the absorption of fat-soluble nutrients. In the HoFH clinical trial, patients were provided daily dietary supplements of vitamin E, linoleic acid, alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). In this trial, the median levels of serum vitamin E, ALA, linoleic acid, EPA, DHA, and arachidonic acid decreased from baseline to Week 26 but remained above the lower limit of the reference range. Adverse clinical consequences of these reductions were not observed with JUXTAPID treatment of up to 78 weeks. Patients treated with JUXTAPID should take daily supplements that contain 400 international units vitamin E and at least 200 mg linoleic acid, 210 mg ALA, 110 mg EPA, and 80 mg DHA [see Dosage and Administration (2.1)]. Patients with chronic bowel or pancreatic diseases that predispose to malabsorption may be at increased risk for deficiencies in these nutrients with use of JUXTAPID.
5.5 Gastrointestinal Adverse Reactions
Gastrointestinal adverse reactions were reported by 27 (93%) of 29 patients in the HoFH clinical trial. Diarrhea occurred in 79% of patients, nausea in 65%, dyspepsia in 38%, and vomiting in 34%. Other reactions reported by at least 20% of patients include abdominal pain, abdominal discomfort, abdominal distension, constipation, and flatulence [see Adverse Reactions (6)].
Gastrointestinal adverse reactions of severe intensity were reported by 6 (21%) of 29 patients in the HoFH clinical trial, with the most common being diarrhea (4 patients, 14%); vomiting (3 patients, 10%); and abdominal pain, distension, and/or discomfort (2 patients, 7%). Gastrointestinal reactions contributed to the reasons for early discontinuation from the trial for 4 (14%) patients.
There have been postmarketing reports of severe diarrhea with the use of JUXTAPID, including patients being hospitalized because of diarrhea-related complications such as volume depletion. Monitor patients who are more susceptible to complications from diarrhea, such as older patients and patients taking drugs that can lead to volume depletion or hypotension. Instruct patients to stop JUXTAPID and contact their healthcare provider if severe diarrhea occurs or if they experience symptoms of volume depletion such as lightheadedness, decreased urine output, or tiredness. In such cases, consider reducing the dose or suspending use of JUXTAPID.
Absorption of concomitant oral medications may be affected in patients who develop diarrhea or vomiting.
To reduce the risk of gastrointestinal adverse events, patients should adhere to a low-fat diet supplying <20% of energy from fat and the dosage of JUXTAPID should be increased gradually [see Dosage and Administration (2.1) and (2.2)].
5.6 Concomitant Use of CYP3A4 Inhibitors
CYP3A4 inhibitors increase the exposure of lomitapide, with strong inhibitors increasing exposure approximately 27-fold. Concomitant use of moderate or strong CYP3A4 inhibitors with JUXTAPID is contraindicated [see Drug Interactions (7.1)]. In the JUXTAPID clinical trials, one patient with HoFH developed markedly elevated transaminases (ALT 24× ULN, AST 13× ULN) within days of initiating the strong CYP3A4 inhibitor clarithromycin. If treatment with moderate or strong CYP3A4 inhibitors is unavoidable, JUXTAPID should be stopped during the course of treatment.
Grapefruit juice must be omitted from the diet while being treated with JUXTAPID.
Weak CYP3A4 inhibitors can increase the exposure of lomitapide approximately 2-fold; therefore, when JUXTAPID is administered with weak CYP3A4 inhibitors, the dose of JUXTAPID should be decreased by half. Careful titration may then be considered based on LDL-C response and safety/tolerability to a maximum recommended dosage of 30 mg daily except when coadministered with oral contraceptives, in which case the maximum recommended lomitapide dosage is 40 mg daily [see Dosage and Administration (2.3) and Drug Interactions (7.2)].
5.7 Risk of Myopathy with Concomitant Use of Simvastatin or Lovastatin
The risk of myopathy, including rhabdomyolysis, with simvastatin and lovastatin monotherapy is dose related. Lomitapide approximately doubles the exposure to simvastatin; therefore, it is recommended to reduce the dose of simvastatin by 50% when initiating JUXTAPID [see Clinical Pharmacology (12.3)]. While taking JUXTAPID, limit simvastatin dosage to 20 mg daily (or 40 mg daily for patients who have previously tolerated simvastatin 80 mg daily for at least one year without evidence of muscle toxicity). Refer to the simvastatin prescribing information for additional dosing recommendations.
Interaction between lovastatin and lomitapide has not been studied. However, the metabolizing enzymes and transporters responsible for the disposition of lovastatin and simvastatin are similar, suggesting that JUXTAPID may increase the exposure of lovastatin; therefore, reducing the dose of lovastatin should be considered when initiating JUXTAPID.
5.8 Risk of Supratherapeutic or Subtherapeutic Anticoagulation with Warfarin
JUXTAPID increases the plasma concentrations of warfarin. Increases in the dose of JUXTAPID may lead to supratherapeutic anticoagulation, and decreases in the dose of JUXTAPID may lead to subtherapeutic anticoagulation. Difficulty controlling INR contributed to early discontinuation from the HoFH clinical trial for one of five patients taking concomitant warfarin. Patients taking warfarin should undergo regular monitoring of the INR, especially after any changes in JUXTAPID dosage. The dose of warfarin should be adjusted as clinically indicated [see Drug Interactions (7.3)].
-
6 ADVERSE REACTIONS
The following important adverse reactions have been observed and are discussed in detail in other sections of the label:
- Risk of hepatotoxicity [see Warnings and Precautions (5.1)]
- Reduced absorption of fat-soluble vitamins, and serum fatty acids [see Warnings and Precautions (5.4)]
- Gastrointestinal adverse reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
One single-arm, open-label, 78-week trial has been conducted in 29 patients with HoFH, 23 of whom completed at least one year of treatment. The initial dosage of JUXTAPID was 5 mg daily, with titration up to 60 mg daily during an 18-week period based on safety and tolerability. In this trial, the mean age was 30.7 years (range, 18 to 55 years), 16 (55%) patients were men, 25 (86%) patients were Caucasian, 2 (7%) were Asian, 1 (3%) was African American, and 1 (3%) was multi-racial [see Clinical Studies (14)].
Five (17%) of the 29 patients with HoFH that participated in the clinical trial discontinued treatment due to an adverse reaction. The adverse reactions that contributed to treatment discontinuations included diarrhea (2 patients; 7%) and abdominal pain, nausea, gastroenteritis, weight loss, headache, and difficulty controlling INR on warfarin (1 patient each; 3%).
The most common adverse reactions were gastrointestinal, reported by 27 (93%) of 29 patients. Adverse reactions reported by ≥8 (28%) patients in the HoFH clinical trial included diarrhea, nausea, vomiting, dyspepsia, and abdominal pain. Other common adverse reactions, reported by 5 to 7 (17-24%) patients, included weight loss, abdominal discomfort, abdominal distension, constipation, flatulence, increased ALT, chest pain, influenza, nasopharyngitis, and fatigue.
The adverse reactions reported in at least 10% of patients during the HoFH clinical trial are presented in Table 4.
Table 4: Adverse Reactions Reported in ≥10% of Patients in the Clinical Trial in HoFH ADVERSE REACTION N (%) Gastrointestinal Disorders Diarrhea 23 (79) Nausea 19 (65) Dyspepsia 11 (38) Vomiting 10 (34) Abdominal pain 10 (34) Abdominal discomfort 6 (21) Abdominal distension 6 (21) Constipation 6 (21) Flatulence 6 (21) Gastroesophageal reflux disease 3 (10) Defecation urgency 3 (10) Rectal tenesmus 3 (10) Infections Influenza 6 (21) Nasopharyngitis 5 (17) Gastroenteritis 4 (14) Investigations Decreased weight 7 (24) Increased ALT 5 (17) General Disorders Chest pain 7 (24) Fatigue 5 (17) Fever 3 (10) Musculoskeletal Disorders Back pain 4 (14) Nervous System Disorders Headache 3 (10) Dizziness 3 (10) Respiratory Disorders Pharyngolaryngeal pain 4 (14) Nasal congestion 3 (10) Cardiac Disorders Angina pectoris 3 (10) Palpitations 3 (10) Adverse reactions of severe intensity were reported by 8 (28%) of 29 patients, with the most common being diarrhea (4 patients, 14%), vomiting (3 patients, 10%), increased ALT or hepatotoxicity (3 patients, 10%), and abdominal pain, distension, and/or discomfort (2 patients, 7%).
Transaminase Elevations
During the HoFH clinical trial, 10 (34%) of 29 patients had at least one elevation in ALT and/or AST ≥3× ULN (see Table 5). No clinically meaningful elevations in total bilirubin or alkaline phosphatase were observed. Transaminases typically fell within one to four weeks of reducing the dose or withholding JUXTAPID.
Table 5: Patient Incidence of Transaminase Elevations During the HoFH Clinical Trial N (%) Upper limits of normal (ULN) ranged from 33-41 international units/L for ALT and 36-43 international units/L for AST. Total Patients 29 Maximum ALT ≥3 to <5 × ULN 6 (21%) ≥5 to <10 × ULN 3 (10%) ≥10 to <20 × ULN 1 (3%) ≥20 × ULN 0 Maximum AST ≥3 to <5 × ULN 5 (17%) ≥5 to <10 × ULN 1 (3%) ≥10 to <20 × ULN 0 ≥20 × ULN 0 Among the 19 patients who enrolled in an extension study following the HoFH clinical trial, one discontinued because of increased transaminases that persisted despite several dose reductions, and one temporarily discontinued because of markedly elevated transaminases (ALT 24× ULN, AST 13× ULN) that had several possible causes, including a drug-drug interaction between JUXTAPID and the strong CYP3A4 inhibitor clarithromycin [see Drug Interactions (7.1)].
Hepatic Steatosis
Hepatic fat was prospectively measured using magnetic resonance spectroscopy (MRS) in all eligible patients during the HoFH clinical trial. After 26 weeks, the median absolute increase in hepatic fat from baseline was 6%, and the mean absolute increase was 8% (range, 0% to 30%). After 78 weeks, the median absolute increase in hepatic fat from baseline was 6%, and the mean absolute increase was 7% (range, 0% to 18%). Among the 23 patients with evaluable data, on at least one occasion during the trial, 18 (78%) exhibited an increase in hepatic fat >5% and 3 (13%) exhibited an increase >20%. Data from individuals who had repeat measurements after stopping JUXTAPID show that hepatic fat accumulation is reversible, but whether histological sequelae remain is unknown.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of JUXTAPID. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to JUXTAPID exposure.
Musculoskeletal disorders: Myalgia
Skin reactions: Alopecia
-
7 DRUG INTERACTIONS
7.1 Moderate and Strong CYP3A4 Inhibitors
A strong CYP3A4 inhibitor has been shown to increase lomitapide exposure approximately 27-fold [see Clinical Pharmacology (12.3)]. Concomitant use of strong CYP3A4 inhibitors (such as boceprevir, clarithromycin, conivaptan, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, nefazodone, nelfinavir, posaconazole, ritonavir, saquinavir, telaprevir, telithromycin, tipranavir/ritonavir, voriconazole) with lomitapide is contraindicated. Concomitant use of moderate CYP3A4 inhibitors (such as amprenavir, aprepitant, atazanavir, ciprofloxacin, crizotinib, darunavir/ritonavir, diltiazem, erythromycin, fluconazole, fosamprenavir, imatinib, verapamil) has not been studied, but concomitant use with lomitapide is contraindicated since lomitapide exposure will likely increase significantly in the presence of these inhibitors.
Patients must avoid grapefruit juice while taking JUXTAPID [see Contraindications (4), Warnings and Precautions (5.6), and Clinical Pharmacology (12.3)].
7.2 Weak CYP3A4 Inhibitors
Weak CYP3A4 inhibitors (such as alprazolam, amiodarone, amlodipine, atorvastatin, bicalutamide, cilostazol, cimetidine, cyclosporine, fluoxetine, fluvoxamine, ginkgo, goldenseal, isoniazid, lapatinib, nilotinib, pazopanib, ranitidine, ranolazine, ticagrelor, zileuton) can increase lomitapide exposure approximately 2-fold [see Clinical Pharmacology (12.3)]. When administered with weak CYP3A4 inhibitors, the dose of JUXTAPID should be decreased by half. Careful titration of JUXTAPID may then be considered based on LDL-C response and safety/tolerability to a maximum recommended dosage of 30 mg daily except when coadministered with oral contraceptives, in which case the maximum recommended lomitapide dosage is 40 mg daily [see Dosage and Administration (2.3), Warnings and Precautions (5.6), and Clinical Pharmacology (12.3)].
7.3 Warfarin
Lomitapide increases plasma concentrations of both R(+)-warfarin and S(-)-warfarin by approximately 30% and increased the INR 22%. Patients taking warfarin should undergo regular monitoring of INR, particularly after any changes in lomitapide dosage. The dose of warfarin should be adjusted as clinically indicated [see Warnings and Precautions (5.8)].
7.4 Simvastatin and Lovastatin
The risk of myopathy, including rhabdomyolysis, with simvastatin and lovastatin monotherapy is dose related. Lomitapide approximately doubles the exposure of simvastatin; therefore, the recommended dose of simvastatin should be reduced by 50% when initiating JUXTAPID [see Clinical Pharmacology (12.3)]. While taking JUXTAPID, limit simvastatin dosage to 20 mg daily (or 40 mg daily for patients who have previously tolerated simvastatin 80 mg daily for at least one year without evidence of muscle toxicity). Refer to the simvastatin prescribing information for simvastatin dosing recommendations.
Interaction between lovastatin and lomitapide has not been studied. However, the metabolizing enzymes and transporters responsible for the disposition of lovastatin and simvastatin are similar, suggesting that JUXTAPID may increase the exposure of lovastatin; therefore, reducing the dose of lovastatin should be considered when initiating JUXTAPID.
7.5 P-glycoprotein Substrates
Lomitapide is an inhibitor of P-glycoprotein (P-gp). Coadministration of lomitapide with P-gp substrates (such as aliskiren, ambrisentan, colchicine, dabigatran etexilate, digoxin, everolimus, fexofenadine, imatinib, lapatinib, maraviroc, nilotinib, posaconazole, ranolazine, saxagliptin, sirolimus, sitagliptin, talinolol, tolvaptan, topotecan) may increase the absorption of P-gp substrates. Dose reduction of the P-gp substrate should be considered when used concomitantly with lomitapide.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to JUXTAPID during pregnancy. For additional information visit www.JUXTAPID.com or call the Global Lomitapide Pregnancy Exposure Registry (PER) at 1-877-902-4099. Healthcare professionals are encouraged to call the PER at 1-877-902-4099 to enroll patients who become pregnant during JUXTAPID treatment.
Risk Summary
Based on findings from animal studies, JUXTAPID use is contraindicated in pregnancy since it may cause fetal harm [see Contraindications (4), Warnings and Precautions (5.3)]. Available human data are insufficient to draw conclusions about any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes. However, in animal reproduction studies, lomitapide was teratogenic in rats at clinically relevant exposures and in ferrets at exposures estimated to be less than human therapeutic exposure at 60 mg when administered during organogenesis, based on AUC comparisons. Embryo-fetal lethality was observed in rabbits at 6-times the maximum recommended human dose (MRHD) of 60 mg based on body surface area. If pregnancy is detected, discontinue JUXTAPID.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Oral gavage doses of 0.04, 0.4, or 4 mg/kg/day lomitapide given to pregnant rats from gestation day 6 through organogenesis were associated with fetal malformations at ≥2-times human exposure at the MRHD (60 mg) based on plasma AUC comparisons. Fetal malformations included umbilical hernia, gastroschisis, imperforate anus, alterations in heart shape and size, limb malrotations, skeletal malformations of the tail, and delayed ossification of cranial, vertebral and pelvic bones.
Oral gavage doses of 1.6, 4, 10, or 25 mg/kg/day lomitapide given to pregnant ferrets from gestation day 12 through organogenesis were associated with both maternal toxicity and fetal malformations at exposures that ranged from less than the human exposure at the MRHD to 5-times the human exposure at the MRHD. Fetal malformations included umbilical hernia, medially rotated or short limbs, absent or fused digits on paws, cleft palate, open eye lids, low-set ears, and kinked tail.
Oral gavage doses of 0.1, 1, or 10 mg/kg/day lomitapide given to pregnant rabbits from gestation day 6 through organogenesis were not associated with adverse effects at systemic exposures up to 3-times the MRHD of 60 mg based on body surface area comparison. Treatment at doses of ≥20 mg/kg/day, ≥6-times the MRHD, resulted in embryo-fetal lethality.
Pregnant female rats given oral gavage doses of 0.1, 0.3, or 1 mg/kg/day lomitapide from gestation day 7 through termination of nursing on lactation day 20 were associated with malformations at systemic exposures equivalent to human exposure at the MRHD of 60 mg based on AUC. Increased pup mortality occurred at 4-times the MRHD.
8.2 Lactation
Risk Summary
There are no data on the presence of lomitapide in human or animal milk, effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions, including hepatotoxicity, advise patients that breastfeeding is not recommended during treatment with JUXTAPID.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Females of reproductive potential should have a negative pregnancy test before starting JUXTAPID.
Contraception
Based on animal studies, JUXTAPID may cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with JUXTAPID and for two weeks after the final dose.
The use of JUXTAPID may result in reduced efficacy of oral contraceptives if vomiting or diarrhea occurs. Advise patients using oral contraceptives and who experience vomiting or diarrhea to use an effective alternative contraceptive method until 7 days after resolution of symptoms [see Drug Interactions (7.2)].
8.5 Geriatric Use
Clinical studies of JUXTAPID did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dosing for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Patients with end-stage renal disease receiving dialysis should not exceed 40 mg daily since lomitapide exposure in these patients increased approximately 50% compared with healthy volunteers. Effects of mild, moderate, and severe renal impairment, including those with end-stage renal disease not yet receiving dialysis, on lomitapide exposure have not been studied. However, it is possible that patients with renal impairment who are not yet receiving dialysis may experience increases in lomitapide exposure exceeding 50% [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with mild hepatic impairment (Child-Pugh A) should not exceed 40 mg daily since the lomitapide exposure in these patients increased approximately 50% compared with healthy volunteers. JUXTAPID is contraindicated in patients with moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment since the lomitapide exposure in patients with moderate hepatic impairment increased 164% compared with healthy volunteers [see Contraindications (4) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There is no specific treatment in the event of overdose of JUXTAPID. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Liver-related tests should be monitored. Hemodialysis is unlikely to be beneficial given that lomitapide is highly protein bound.
-
11 DESCRIPTION
JUXTAPID capsules contain lomitapide mesylate, a synthetic lipid-lowering agent for oral administration.
The chemical name of lomitapide mesylate is N-(2,2,2-trifluoroethyl)-9-[4-[4-[[[4'-(trifluoromethyl)[1,1'-biphenyl]-2-yl]carbonyl]amino]-1-piperidinyl]butyl]-9H-fluorene-9-carboxamide, methanesulfonate salt. Its structural formula is:
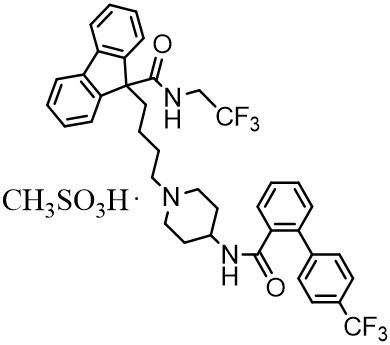
The empirical formula for lomitapide mesylate is C39H37F6N3O2 ∙ CH4O3S and its molecular weight is 789.8.
Lomitapide mesylate is a white to off-white powder that is slightly soluble in aqueous solutions of pH 2 to 5. Lomitapide mesylate is freely soluble in acetone, ethanol, and methanol; soluble in 2-butanol, methylene chloride, and acetonitrile; sparingly soluble in 1-octanol and 2-propanol; slightly soluble in ethyl acetate; and insoluble in heptane.
Each JUXTAPID capsule contains lomitapide mesylate equivalent to 5, 10, 20, or 30 mg lomitapide free base and the following inactive ingredients: pregelatinized starch, sodium starch glycolate, microcrystalline cellulose, lactose monohydrate, silicon dioxide and magnesium stearate. The capsule shells of all strengths contain gelatin and titanium dioxide; the 5 mg, 10 mg and 30 mg capsules also contain red iron oxide; and the 30 mg capsules also contain yellow iron oxide. The imprinting ink contains shellac, black iron oxide, and propylene glycol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
JUXTAPID directly binds and inhibits microsomal triglyceride transfer protein (MTP), which resides in the lumen of the endoplasmic reticulum, thereby preventing the assembly of apo B-containing lipoproteins in enterocytes and hepatocytes. This inhibits the synthesis of chylomicrons and VLDL. The inhibition of the synthesis of VLDL leads to reduced levels of plasma LDL-C.
12.3 Pharmacokinetics
Absorption
Upon oral administration of a single 60-mg dose of JUXTAPID, the lomitapide tmax is around 6 hours in healthy volunteers. The absolute bioavailability of lomitapide is approximately 7%. Lomitapide pharmacokinetics is approximately dose-proportional for oral single doses from 10-100 mg.
Distribution
The mean lomitapide volume of distribution at steady state is 985-1292 liters. Lomitapide is 99.8% plasma-protein bound.
Metabolism
Lomitapide is metabolized extensively by the liver. The metabolic pathways include oxidation, oxidative N-dealkylation, glucuronide conjugation, and piperidine ring opening. Cytochrome P450 (CYP) 3A4 metabolizes lomitapide to its major metabolites, M1 and M3, as detected in plasma. The oxidative N-dealkylation pathway breaks the lomitapide molecule into M1 and M3. M1 is the moiety that retains the piperidine ring, whereas M3 retains the rest of the lomitapide molecule in vitro. CYPs 1A2, 2B6, 2C8, and 2C19 may metabolize lomitapide to a small extent to M1. M1 and M3 do not inhibit activity of microsomal triglyceride transfer protein in vitro.
Excretion
In a mass-balance study, a mean of 59.5% and 33.4% of the dose was excreted in the urine and feces, respectively. In another mass-balance study, a mean of 52.9% and 35.1% of the dose was excreted in the urine and feces, respectively. Lomitapide was not detectable in urine samples. M1 is the major urinary metabolite. Lomitapide is the major component in the feces. The mean lomitapide terminal half-life is 39.7 hours.
Specific Populations
Hepatic Impairment
A single-dose, open-label study was conducted to evaluate the pharmacokinetics of 60 mg lomitapide in healthy volunteers with normal hepatic function compared with patients with mild (Child-Pugh A) and moderate (Child-Pugh B) hepatic impairment. In patients with moderate hepatic impairment, lomitapide AUC and Cmax were 164% and 361% higher, respectively, compared with healthy volunteers. In patients with mild hepatic impairment, lomitapide AUC and Cmax were 47% and 4% higher, respectively, compared with healthy volunteers. Lomitapide has not been studied in patients with severe hepatic impairment (Child-Pugh score 10-15) [see Dosage and Administration (2.6), Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.7)].
Renal Impairment
A single-dose, open-label study was conducted to evaluate the pharmacokinetics of 60 mg lomitapide in patients with end-stage renal disease receiving hemodialysis compared with healthy volunteers with normal renal function. Healthy volunteers had estimated creatinine clearance >80 mL/min by the Cockcroft-Gault equation. Compared with healthy volunteers, lomitapide AUC0-inf and Cmax were 40% and 50% higher, respectively, in patients with end-stage renal disease receiving hemodialysis. Effects of mild, moderate, and severe renal impairment as well as end-stage renal disease not yet on dialysis on lomitapide exposure have not been studied [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)].
Drug Interactions
[see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.6), (5.7), (5.8), and Drug Interactions (7)].
In vitro Assessment of Drug Interactions
Lomitapide does not induce CYPs 1A2, 3A4, or 2B6. Lomitapide inhibits CYP3A4. Lomitapide does not inhibit CYPs 1A2, 2B6, 2C9, 2C19, 2D6, or 2E1. M1 and M3 do not induce CYPs 1A2, 3A4, or 2B6. M1 and M3 do not inhibit CYPs 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4. Lomitapide is not a P-gp substrate. Lomitapide inhibits P-gp but does not inhibit breast cancer resistance protein (BCRP).
Effects of other Drugs on Lomitapide
Table 6 summarizes the effect of coadministered drugs on lomitapide AUC and Cmax.
Table 6: Effect of Coadministered Drugs on Lomitapide Systemic Exposure COADMINISTERED DRUG DOSING OF COADMINISTERED DRUG DOSING OF LOMITAPIDE RATIO OF LOMITAPIDE EXPOSURE WITH/WITHOUT COADMINISTERED DRUG
NO EFFECT = 1AUC Cmax BID = twice daily; QD = once daily ↑ = increase Contraindicated with lomitapide [see Contraindications (4) and Warnings and Precautions (5.6)] Ketoconazole 200 mg BID for 9 days 60 mg single dose ↑ 27 ↑ 15 Adjustment necessary when coadministered with lomitapide [see Dosage and Administration (2.3) and Warnings and Precautions (5.6)] AUC Cmax Atorvastatin 80 mg QD 20 mg single dose ↑2 ↑2.1 Ethinyl Estradiol (EE) / norgestimate 0.035 mg EE/ 0.25 mg norgestimate QD 20 mg single dose ↑1.3 ↑1.4 Effect of Lomitapide on other Drugs
Table 7 summarizes the effects of lomitapide on the AUC and Cmax of coadministered drugs.
Table 7: Effect of Lomitapide on the Systemic Exposure of Coadministered Drugs COADMINISTERED DRUG DOSING OF COADMINISTERED DRUG DOSING OF LOMITAPIDE CHANGE OF COADMINISTERED DRUG EXPOSURE WITH / WITHOUT LOMITAPIDE AUC Cmax QD = once daily; INR = international normalized ratio; ↑ = increase; ↓ = decrease - * Limit simvastatin dosage to 20 mg daily (or 40 mg daily for patients who have previously tolerated simvastatin 80 mg daily for at least one year without evidence of muscle toxicity). Refer to the simvastatin prescribing information for additional dosing recommendations.
- † Patients taking warfarin should undergo regular monitoring of the INR, especially after any changes in lomitapide dosage.
Dosage adjustment necessary when coadministered with lomitapide Simvastatin* 40 mg single dose 60 mg QD × 7 days Simvastatin ↑ 99% ↑ 102% Simvastatin acid ↑ 71% ↑ 57% 20 mg single dose 10 mg QD × 7 days Simvastatin ↑ 62% ↑65% Simvastatin acid ↑ 39% ↑ 35% Warfarin† 10 mg single dose 60 mg QD × 12 days R(+) warfarin ↑ 28% ↑ 14% S(-) warfarin ↑ 30% ↑ 15% INR ↑ 7% ↑ 22% No dosing adjustments required for the following: Atorvastatin 20 mg single dose 60 mg QD × 7 days Atorvastatin acid ↑ 52% ↑63% 20 mg single dose 10 mg QD × 7 days Atorvastatin acid ↑ 11% ↑19% Rosuvastatin 20 mg single dose 60 mg QD × 7 days Rosuvastatin ↑ 32% ↑ 4% 20 mg single dose 10 mg QD × 7 days Rosuvastatin ↑ 2% ↑ 6% Fenofibrate, micronized 145 mg single dose 10 mg QD × 7 days Fenofibric acid ↓ 10% ↓29% Ezetimibe 10 mg single dose 10 mg QD × 7 days Total ezetimibe ↑ 6% ↑ 3% Extended release niacin 1000 mg single dose 10 mg QD × 7 days Nicotinic acid ↑ 10% ↑ 11% Nicotinuric acid ↓ 21% ↓ 15% Ethinyl estradiol 0.035 mg QD × 28 days 50 mg QD × 8 days Ethinyl estradiol ↓ 8% ↓ 8% Norgestimate 0.25 mg QD × 28 days 50 mg QD × 8 days 17-Deacetyl norgestimate ↑ 6% ↑ 2% -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dietary carcinogenicity study in mice, lomitapide was administered at doses of 0.3, 1.5, 7.5, 15, or 45 mg/kg/day. There were statistically significant increases in the incidences of liver adenomas and carcinomas in males at doses ≥1.5 mg/kg/day (≥2-times the MRHD at 60 mg based on AUC) and in females at ≥7.5 mg/kg/day (≥10-times the human exposure at 60 mg based on AUC). Incidences of small intestinal carcinomas in males and combined adenomas and carcinomas in females were significantly increased at doses ≥15 mg/kg/day (≥23-times the human exposure at 60 mg based on AUC).
In a 2-year carcinogenicity study in rats, lomitapide was administered by oral gavage for up to 99 weeks at doses of 0.25, 1.7, or 7.5 mg/kg/day in males and 0.03, 0.35, or 2.0 mg/kg/day in females. While the design of the study was suboptimal, there were no statistically significant drug-related increases in tumor incidences at exposures up to 6-times (males) and 8-times (females) higher than human exposure at the MRHD based on AUC.
Lomitapide did not exhibit genotoxic potential in a battery of studies, including the in vitro Bacterial Reverse Mutation (Ames) assay, an in vitro cytogenetics assay using primary human lymphocytes, and an oral micronucleus study in rats.
Lomitapide had no effect on fertility in rats at doses up to 5 mg/kg/day at systemic exposures estimated to be 4-times (females) and 5-times (males) higher than in humans at 60 mg based on AUC.
-
14 CLINICAL STUDIES
The safety and effectiveness of JUXTAPID as an adjunct to a low-fat diet and other lipid-lowering treatments, including LDL apheresis where available, were evaluated in a multinational, single-arm, open-label, 78-week trial involving 29 adults with HoFH. A diagnosis of HoFH was defined by the presence of at least one of the following clinical criteria: (1) documented functional mutation(s) in both LDL receptor alleles or alleles known to affect LDL receptor functionality, or (2) skin fibroblast LDL receptor activity <20% normal, or (3) untreated TC >500 mg/dL and TG <300 mg/dL and both parents with documented untreated TC >250 mg/dL.
Among the 29 patients enrolled, the mean age was 30.7 years (range, 18 to 55 years), 16 (55%) were men, and the majority (86%) were Caucasian. The mean body mass index (BMI) was 25.8 kg/m2, with four patients meeting BMI criteria for obesity; one patient had type 2 diabetes. Concomitant lipid-lowering treatments at baseline included one or more of the following: statins (93%), ezetimibe (76%), nicotinic acid (10%), bile acid sequestrant (3%), and fibrate (3%); 18 (62%) were receiving apheresis.
After a six-week run-in period to stabilize lipid-lowering treatments, including the establishment of an LDL apheresis schedule if applicable, JUXTAPID was initiated at 5 mg daily and titrated to daily doses of 10 mg, 20 mg, 40 mg, and 60 mg at weeks 2, 6, 10, and 14, respectively, based on tolerability and acceptable levels of transaminases. Patients were instructed to maintain a low-fat diet (<20% calories from fat) and to take dietary supplements that provided approximately 400 international units vitamin E, 210 mg alpha-linolenic acid (ALA), 200 mg linoleic acid, 110 mg eicosapentaenoic acid (EPA), and 80 mg docosahexaenoic acid (DHA) per day. After efficacy was assessed at Week 26, patients remained on JUXTAPID for an additional 52 weeks to assess long-term safety. During this safety phase, the dose of JUXTAPID was not increased above each patient's maximum tolerated dose established during the efficacy phase, but changes to concomitant lipid-lowering treatments were allowed.
Twenty-three (79%) patients completed the efficacy endpoint at Week 26, all of whom went on to complete 78 weeks of treatment. Adverse events contributed to premature discontinuation for five patients [see Adverse Reactions (6.1)]. The maximum tolerated doses during the efficacy period were 5 mg (10%), 10 mg (7%), 20 mg (21%), 40 mg (24%), and 60 mg (34%).
The primary efficacy endpoint was percent change in LDL-C from baseline to Week 26. At Week 26, the mean and median percent changes in LDL-C from baseline were -40% (paired t-test p<0.001) and -50%, respectively, based on the intent-to-treat population with last observation carried forward (LOCF) for patients who discontinued prematurely. The mean percent change in LDL-C from baseline through Week 26 is shown in Figure 1 for the 23 patients who completed the efficacy period.
Figure 1: Mean Percent Change in LDL-C from Baseline (Week 26 Completers) Error bars represent 95% confidence intervals of the mean. 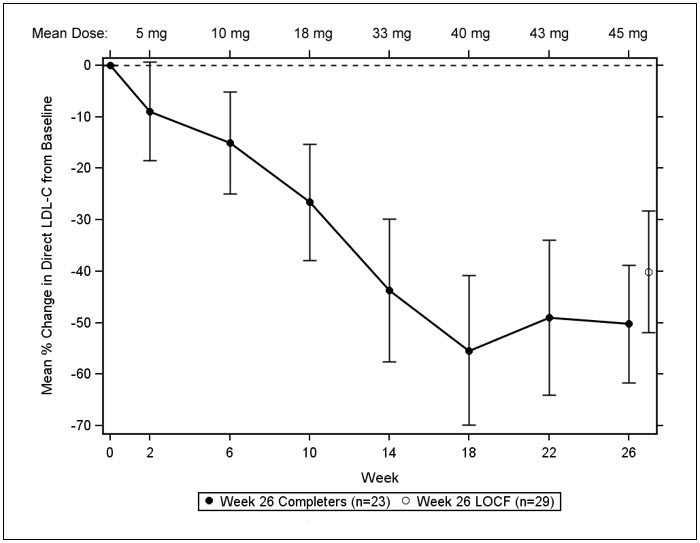
Changes in lipids and lipoproteins through the efficacy endpoint at Week 26 are presented in Table 8.
Table 8: Absolute Values and Percent Changes from Baseline in Lipids and Lipoproteins PARAMETER BASELINE WEEK 26/LOCF (N=29) Mean (SD) Mean (SD) Mean % Change - * Statistically significant compared with baseline based on the pre-specified gatekeeping method for controlling Type I error among the primary and key secondary endpoints.
- † Median values with interquartile range and median % change presented for TG.
LDL-C, direct (mg/dL) 336 (114) 190 (104) -40 * TC (mg/dL) 430 (135) 258 (118) -36 * apo B (mg/dL) 259 (80) 148 (74) -39 * Non-HDL-C (mg/dL) 386 (132) 217 (113) -40 VLDL-C (mg/dL) 21 (10) 13 (9) -29 TG (mg/dL)† 92 [72, 128] 57 [36, 78] -45 * HDL-C (mg/dL) 44 (11) 41 (13) -7 After Week 26, during the safety phase of the study, adjustments to concomitant lipid-lowering treatments were allowed. For the study population overall, average reductions in LDL-C, TC, apo B, and non-HDL-C were sustained during chronic therapy.
-
16 HOW SUPPLIED / STORAGE AND HANDLING
5 mg capsules:
Orange/orange hard gelatin capsule printed with black ink "A733" and "5 mg"
Bottles of 28 NDC: 10122-405-28 10 mg capsules:
Orange/white hard gelatin capsule printed with black ink "A733" and "10 mg"
Bottles of 28 NDC: 10122-410-28 20 mg capsules:
White/white hard gelatin capsule printed with black ink "A733" and "20 mg"
Bottles of 28 NDC: 10122-420-28 30 mg capsules:
Orange/yellow hard gelatin capsule printed with black ink "A733" and "30 mg"
Bottles of 28 NDC: 10122-430-28 Storage: Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized. Keep container tightly closed and protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved labeling (Medication Guide)
Patients should be informed that a registry for patients taking JUXTAPID has been established in order to monitor and evaluate the long-term effects of JUXTAPID. Patients are encouraged to participate in the registry and should be informed that their participation is voluntary. For information regarding the registry program visit www.JUXTAPID.com or call 1-877-902-4099.
Advise patients of the following:
Risk of Hepatotoxicity [see Warnings and Precautions (5.1)]
- JUXTAPID can cause both elevations in transaminases and hepatic steatosis. Discuss with the patient the importance of monitoring of liver-related tests before taking JUXTAPID, prior to each dose escalation, and periodically thereafter.
- Patients should be advised of the potential for increased risk of liver injury if alcohol is consumed while taking JUXTAPID. It is recommended that patients taking JUXTAPID limit consumption to not more than one alcoholic drink per day.
- JUXTAPID is commonly associated with nausea, vomiting, and abdominal pain. Advise patients to promptly report these symptoms if they increase in severity, persist, or change in the character, as they might reflect liver injury. Patients should also report any other symptoms of possible liver injury, including fever, jaundice, lethargy, or flu-like symptoms.
JUXTAPID REMS Program [see Warnings and Precautions (5.2)]
- JUXTAPID is only available through a restricted program called JUXTAPID REMS Program and therefore, JUXTAPID is only available from certified pharmacies that are enrolled in the program.
Embryofetal Toxicity [see Warnings and Precautions (5.3), Drug Interactions (7.2), Use in Specific Populations (8.1, 8.3)]
- JUXTAPID is contraindicated in pregnancy since it may cause fetal harm. Advise females who become pregnant to discontinue JUXTAPID and inform their healthcare provider of a known or suspected pregnancy.
- Advise females of reproductive potential to use effective contraception during treatment with JUXTAPID and for two weeks after the final dose.
- Advise patients who are taking oral contraceptives and experience vomiting or diarrhea while taking JUXTAPID to use an effective alternative contraceptive method until 7 days after resolution of symptoms.
Lactation [see Use in Specific Populations (8.2)]
Advise females not to breastfeed during treatment with JUXTAPID.
Dietary Supplements [see Warnings and Precautions (5.4)]
- Discuss with the patient the importance of taking daily supplements that contain 400 international units vitamin E and at least 200 mg linoleic acid, 210 mg alpha-linolenic acid (ALA), 110 mg eicosapentaenoic acid (EPA), and 80 mg docosahexaenoic acid (DHA).
Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.5)]
- Inform the patient that gastrointestinal adverse reactions are common with JUXTAPID. These include, but are not limited to, diarrhea, nausea/vomiting, abdominal pain/discomfort, flatulence, and constipation. Strict adherence to a low-fat diet (<20% of total calories from fat) may reduce these reactions.
- Instruct the patient to stop JUXTAPID and contact their healthcare provider if severe diarrhea occurs or if they experience symptoms of volume depletion such as lightheadedness, decreased urine output, or tiredness.
- Tell the patient that taking JUXTAPID with food may adversely impact gastrointestinal tolerability; therefore, they should take JUXTAPID at least 2 hours after the evening meal, swallowing each capsule whole.
- Absorption of oral medications may be affected in patients who develop diarrhea or vomiting. For example, hormone absorption from oral contraceptives may be incomplete, warranting the use of additional contraceptive methods. Patients who develop these symptoms should seek advice from their healthcare provider.
Drug Interactions [see Warnings and Precautions (5.6) and Drug Interactions (7)]
- Tell the patient to omit grapefruit juice from his/her diet while on JUXTAPID.
- Because multiple drug-drug interactions have been described with JUXTAPID, advise the patient to tell their healthcare provider(s) about all medications, nutritional supplements, and vitamins that they are taking or may be taking while taking JUXTAPID.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Medication Guide
JUXTAPID® (JUKS-tuh-pid)
(lomitapide)
capsulesWhat is the most important information I should know about JUXTAPID?
- JUXTAPID is available only through certified pharmacies that are enrolled in the JUXTAPID REMS Program. Your doctor must be enrolled in the program in order for you to be prescribed JUXTAPID.
- There is a registry that collects information about the effects of taking JUXTAPID over time. Ask your doctor for more information about this registry or visit www.JUXTAPID.com or call 1-877-902-4099.
1. Liver problems. JUXTAPID can cause liver problems such as increased liver enzymes or increased fat in the liver.- Your doctor should do blood tests to check your liver before you start JUXTAPID, if your dose is increased, and while you take JUXTAPID. If your tests show some liver problems, your doctor may adjust your dose of JUXTAPID or stop it altogether.
- Tell your doctor if you have had liver problems, including liver problems while taking other medicines.
- JUXTAPID may cause nausea, vomiting and stomach pain, especially if you do not eat a low-fat diet. These side effects can also be symptoms of liver problems.
- Tell your doctor right away if you have any of these symptoms of liver problems while taking JUXTAPID:
- Nausea, vomiting, or stomach pain that gets worse, does not go away, or changes
- fever
- flu-like symptoms
- yellowing of your eyes or skin
- you are more tired than usual
- Drinking alcohol may increase your chance of having liver problems or make your liver problems worse. You should not have more than 1 alcoholic drink each day while taking JUXTAPID.
2. Harm to your unborn baby. JUXTAPID may cause harm to your unborn baby.
- If you are pregnant, think you may be pregnant, or are planning to become pregnant, do not take JUXTAPID.
- If you are a female who can get pregnant, you should have a pregnancy test before you start taking JUXTAPID. Your pregnancy test must be negative for you to get JUXTAPID.
- Do not have sex while taking JUXTAPID unless you are using effective birth control.
- Talk with your doctor or nurse to find the best method of birth control for you.
- Birth control pills may not work as well if you have diarrhea or vomiting.
- If you start taking birth control pills while you are taking JUXTAPID, tell your doctor. Your doctor might need to change your dose of JUXTAPID.
- If you become pregnant while taking JUXTAPID, stop taking JUXTAPID and call your doctor right away.
- Pregnancy Exposure Registry: There is a pregnancy exposure registry that monitors outcomes in women exposed to JUXTAPID during pregnancy. If you become pregnant while taking JUXTAPID, call 1-877-902-4099 or visit www.JUXTAPID.com for more information about the JUXTAPID pregnancy exposure registry.
What is JUXTAPID? JUXTAPID is a prescription medicine used along with diet and other lipid-lowering treatments, including low-density lipoprotein (LDL) apheresis where available, in people with homozygous familial hypercholesterolemia (HoFH) to reduce:
- LDL ("bad") cholesterol
- total cholesterol
- a protein that carries "bad" cholesterol in the blood (apolipoprotein B)
- non-high-density lipoprotein cholesterol (non-HDL-C)
It is not known if JUXTAPID can decrease problems from high cholesterol, such as heart attack, stroke, death or other health problems.
It is not known if JUXTAPID is safe and effective in people with high cholesterol who do not have HoFH, including those with heterozygous familial hypercholesterolemia (HeFH).
JUXTAPID should not be taken with certain medicines or food. You should not drink grapefruit juice. Ask your doctor or pharmacist to make sure you can take JUXTAPID with your other medicines, and tell your doctor about any new medicines you might take even for only a short time.
It is not known if JUXTAPID is safe and effective in people with kidney problems including people with end-stage kidney disease who are not on dialysis.
It is not known if JUXTAPID is safe and effective when used in children under the age of 18.
Who should not take JUXTAPID?
Do not take JUXTAPID if you:
- are pregnant, think you may be pregnant, or are planning to become pregnant. See "What is the most important information I should know about JUXTAPID?"
- take medicines that affect how the body breaks down JUXTAPID (that is, strong or moderate CYP3A4 inhibitors). Check with your doctor and/or pharmacist to see if you are taking any of these medications. These may include certain medications intended to treat bacterial, fungal or viral infections, and medications to treat depression, high blood pressure, or angina.
- drink grapefruit juice
- have moderate or severe liver problems or active liver disease, including people who have unexplained abnormal liver tests.
What should I tell my doctor before taking JUXTAPID?
Before you take JUXTAPID, tell your doctor if you:
- have liver problems
- have kidney problems
- have intestine or bowel problems
- drink alcohol
- are breastfeeding or plan to breastfeed. It is not known if JUXTAPID passes into your breast milk. You and your doctor should decide if you will take JUXTAPID or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Before starting a new medicine while taking JUXTAPID, even if you will only be taking it for a short time, ask your doctor or pharmacist if it is safe to take with JUXTAPID.
JUXTAPID may affect the way other medicines work, and other medicines may affect how JUXTAPID works.
Certain medicines can affect how your liver breaks down other medicines.
Especially tell your doctor if you take:
- a blood thinner called warfarin
- medicines for high cholesterol, including statins such as atorvastatin or simvastatin, and resins such as colesevelam or cholestyramine
- medicines for bacteria, fungus, or viral infection (including HIV and hepatitis C)
- medicines for depression, high blood pressure, or angina
- birth control pills
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take JUXTAPID?
- Take JUXTAPID exactly as your doctor tells you to take it.
- Your doctor will tell you how much JUXTAPID to take and when to take it.
- Your doctor may change your dose of JUXTAPID if needed.
- Do not change your JUXTAPID dose yourself.
- Take JUXTAPID 1 time each day at least 2 hours after your evening meal.
- Take JUXTAPID with water.
- You should not take JUXTAPID with food. Taking JUXTAPID with food may cause stomach problems.
- Take JUXTAPID capsules whole. Do not open, crush, dissolve, or chew capsules before swallowing. If you cannot swallow JUXTAPID capsules whole, tell your doctor. You may need a different medicine.
- If you take a medicine that lowers cholesterol by binding bile acids, such as colesevelam or cholestyramine, take it at least 4 hours before or 4 hours after you take JUXTAPID. Ask your doctor if you are not sure if you take these medicines.
- To help lower the chance of stomach problems, stay on a low-fat diet. Ask your doctor about talking to a dietician to learn what you should eat while taking JUXTAPID. JUXTAPID makes it harder for some nutrients to get into your body. Take Vitamin E and fatty acids each day while you take JUXTAPID. Ask your doctor, nurse, or dietician how to add them to your diet.
- If you take too much JUXTAPID, call your doctor right away.
- Do not stop JUXTAPID unless your doctor tells you to stop it.
- If you miss a dose of JUXTAPID, take your usual dose the next day at the usual time. If you stop taking JUXTAPID for more than a week, talk to your doctor before restarting treatment.
What are the possible side effects of JUXTAPID?
JUXTAPID can cause serious side effects, including:
- See "What is the most important information I should know about JUXTAPID?"
- problems absorbing certain nutrients. JUXTAPID may decrease your ability to absorb fat-soluble nutrients such as Vitamin E and fatty acids. You should take supplements each day that contain fat-soluble vitamins. People with bowel or pancreas problems may have an increased chance of not being able to absorb these nutrients. See "How should I take JUXTAPID?"
- gastrointestinal symptoms. Diarrhea, nausea, vomiting, and stomach pain or discomfort are very common when taking JUXTAPID. Strictly following a low-fat diet may help lower the chance of having these symptoms. Stop taking Juxtapid and tell your doctor if you have severe diarrhea, especially if you also have lightheadedness, decreased urine output, or tiredness.
- increased levels of certain blood thinners. JUXTAPID can increase the level of the blood thinner, warfarin. If you take warfarin, your doctor should check your blood clotting times frequently, especially after your dose of JUXTAPID changes.
- liver problems caused by certain drugs. Certain medicines can cause liver problems, including isotretinoin, acetaminophen, methotrexate, tetracyclines, and tamoxifen. If you take these medicines with JUXTAPID your doctor may do blood tests more often to check your liver.
The most common side effects of JUXTAPID include:
- diarrhea
- nausea
- vomiting
- indigestion
- stomach cramping/pain
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of JUXTAPID. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store JUXTAPID?
- Store JUXTAPID at room temperature, between 68°F and 77F° (20°C and 25°C).
- Keep JUXTAPID in a tightly closed container.
- Keep JUXTAPID capsules dry.
- Safely throw away medicine that is out of date or no longer needed.
Keep JUXTAPID and all medicines out of the reach of children.
General information about the safe and effective use of JUXTAPID.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use JUXTAPID for a condition for which it was not prescribed. Do not give JUXTAPID to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about JUXTAPID. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about JUXTAPID that is written for healthcare professionals.
For more information, go to www.JUXTAPID.com or call 1-85-JUXTAPID (1-855-898-2743).
What are the ingredients in JUXTAPID?
Active ingredient: lomitapide mesylate
Inactive ingredients: pregelatinized starch, sodium starch glycolate, microcrystalline cellulose, lactose monohydrate, silicon dioxide, magnesium stearate
Capsule shell: The capsule shells of all strengths contain gelatin and titanium dioxide; the 5 mg, 10 mg and 30 mg capsules also contain red iron oxide; and the 30 mg capsules also contain yellow iron oxide. The imprinting ink contains shellac, black iron oxide, and propylene glycol.
Chiesi
JUXTAPID is a registered trademark owned by the Chiesi Group ©2024 Chiesi USA. All rights reserved.
Manufactured for: Chiesi Farmaceutici S.p.A., Parma, Italy.
This Medication Guide has been approved by the U.S. Food and Drug Administration. 01/2024
70015629-01
7465-00
- PRINCIPAL DISPLAY PANEL - 5 mg Capsule Bottle Label
-
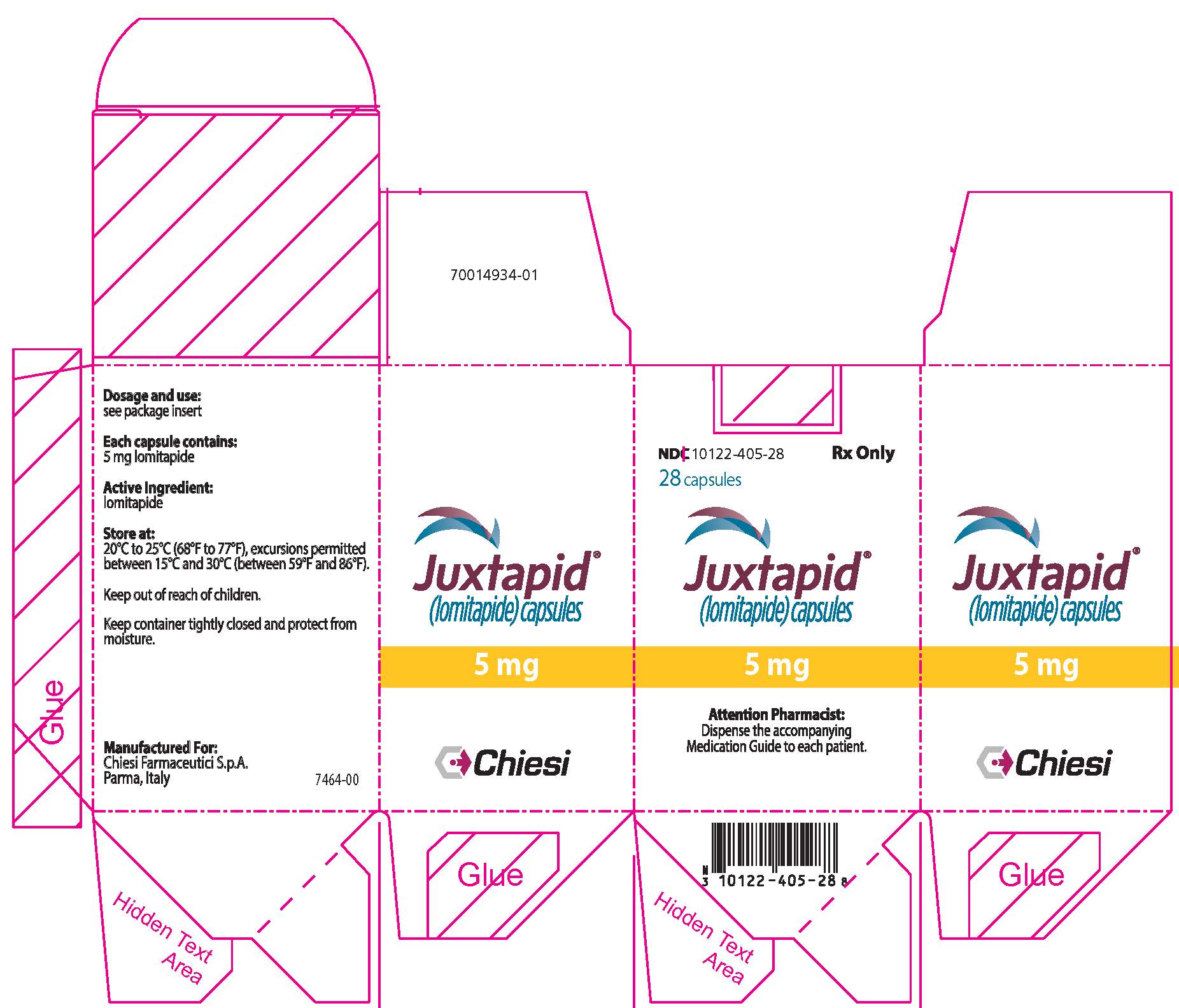
PRINCIPAL DISPLAY PANEL - 5 mg Capsule Bottle Carton
NDC: 10122-405-28
Rx Only28 capsules
Juxtapid®
(lomitapide) capsules5 mg
Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient.Chiesi
- PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Label
-
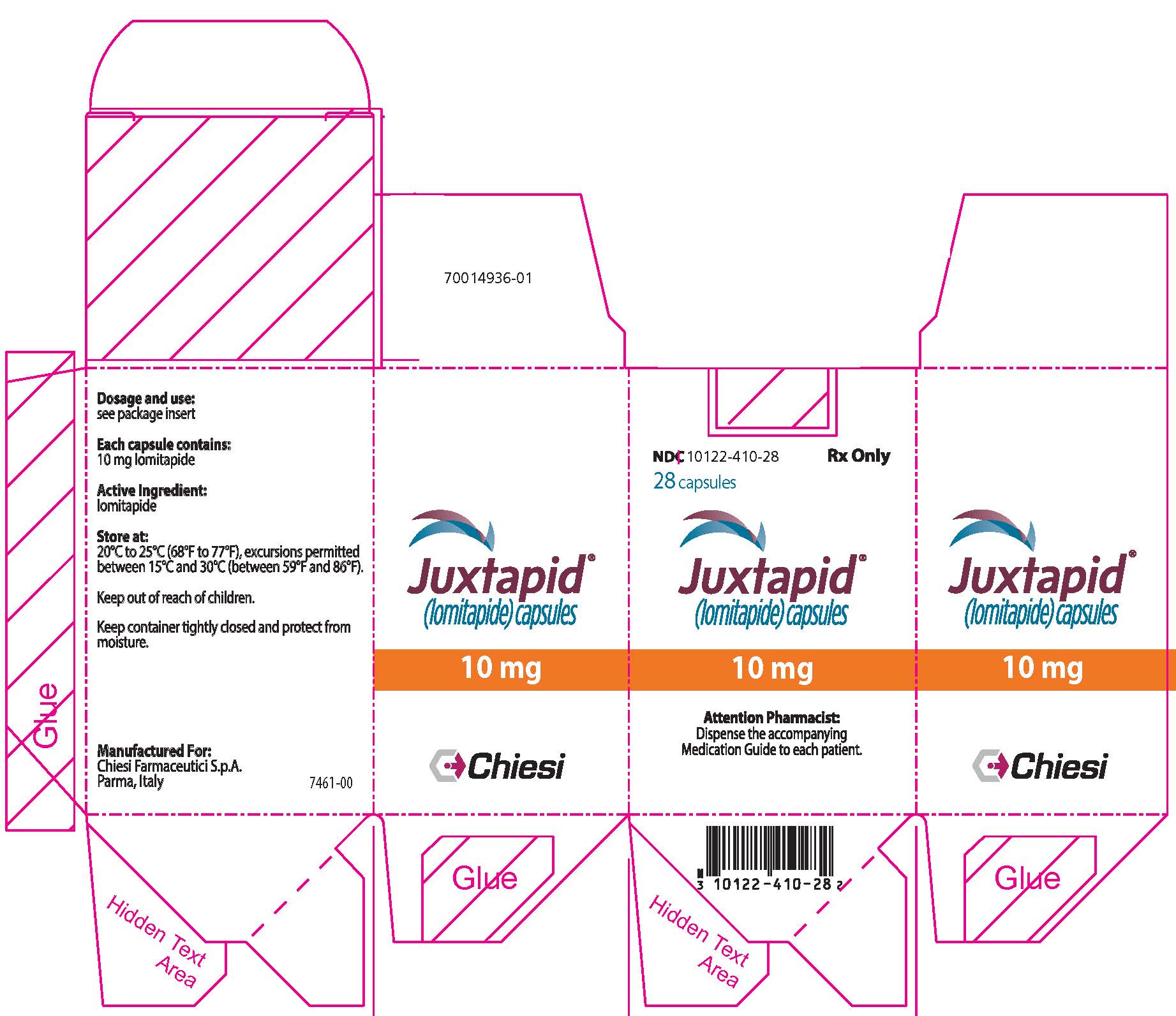
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Carton
NDC: 10122-410-28
Rx Only28 capsules
Juxtapid®
(lomitapide) capsules10 mg
Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient.Chiesi
- PRINCIPAL DISPLAY PANEL - 20 mg Capsule Bottle Label
-
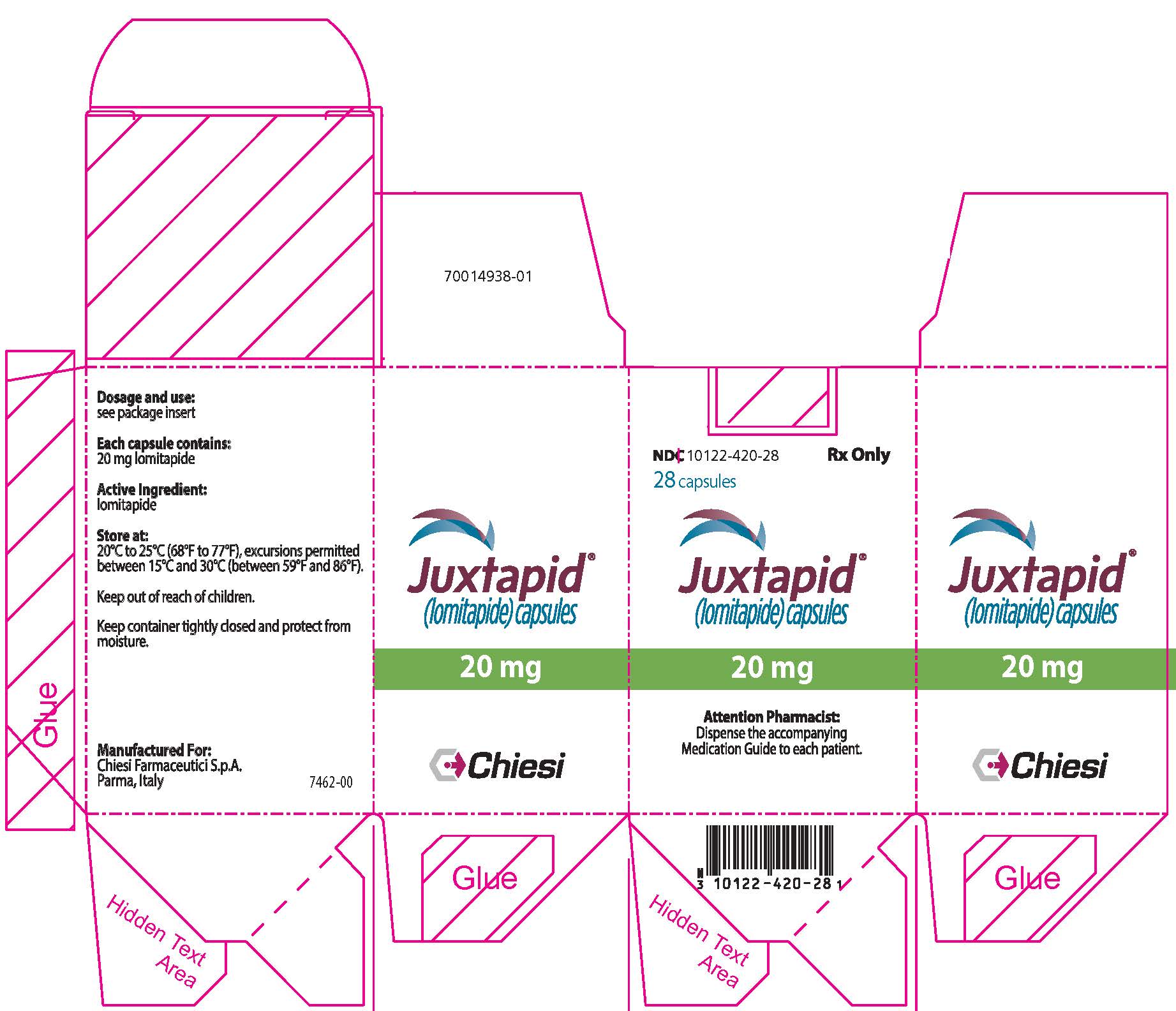
PRINCIPAL DISPLAY PANEL - 20 mg Capsule Bottle Carton
NDC: 10122-420-28
Rx Only28 capsules
Juxtapid®
(lomitapide) capsules20 mg
Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient.Chiesi
- PRINCIPAL DISPLAY PANEL - 30 mg Capsule Bottle Label
-
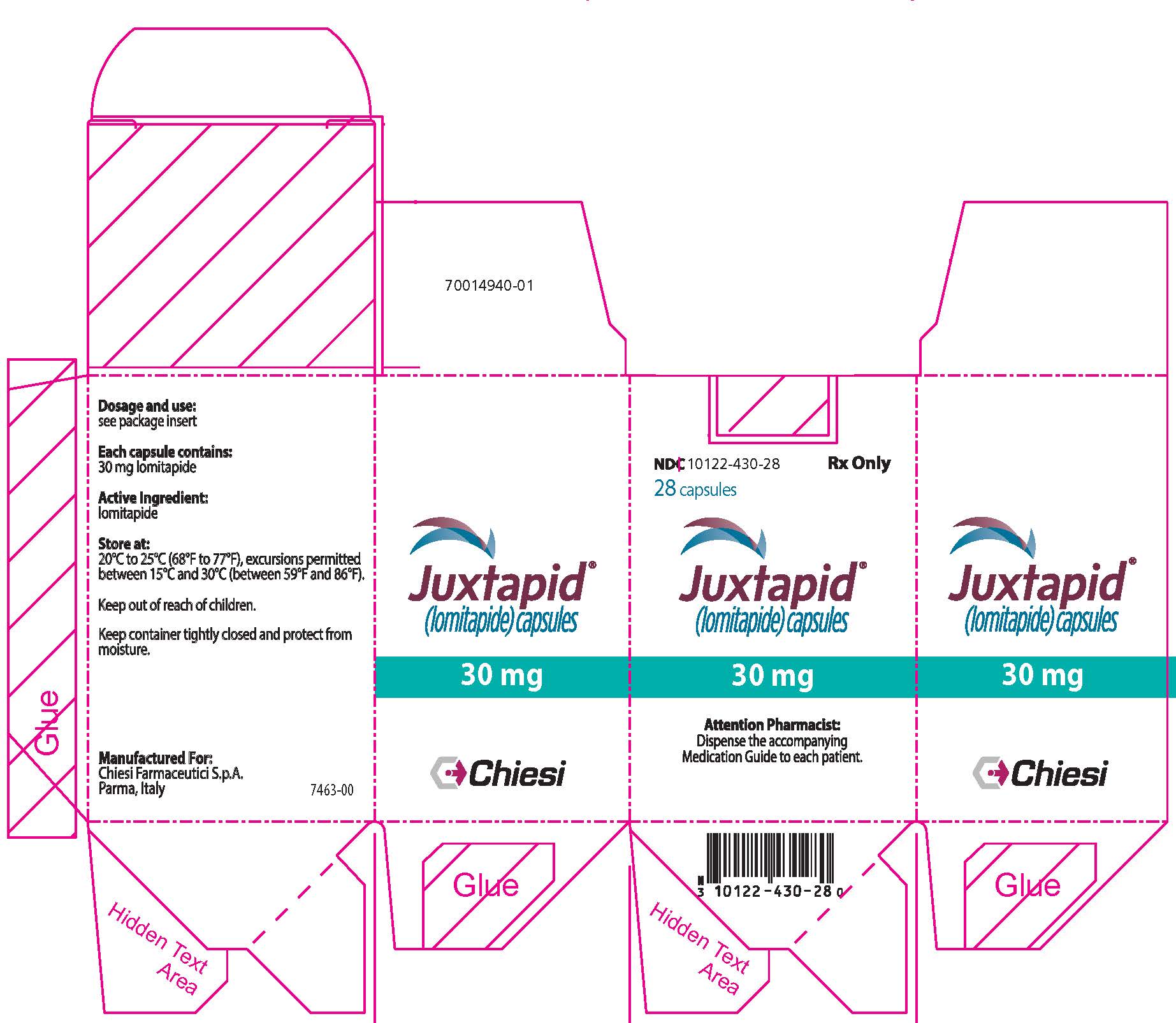
PRINCIPAL DISPLAY PANEL - 30 mg Capsule Bottle Carton
NDC: 10122-430-28
Rx Only28 capsules
Juxtapid®
(lomitapide) capsules30 mg
Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient.Chiesi
-
INGREDIENTS AND APPEARANCE
JUXTAPID
lomitapide mesylate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10122-405 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOMITAPIDE MESYLATE (UNII: X4S83CP54E) (LOMITAPIDE - UNII:82KUB0583F) LOMITAPIDE 5 mg Product Characteristics Color orange (Swedish Orange) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code A733;5mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10122-405-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 01/28/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203858 01/03/2013 JUXTAPID
lomitapide mesylate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10122-410 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOMITAPIDE MESYLATE (UNII: X4S83CP54E) (LOMITAPIDE - UNII:82KUB0583F) LOMITAPIDE 10 mg Product Characteristics Color orange (Swedish Orange) , white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code A733;10mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10122-410-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 01/28/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203858 01/03/2013 JUXTAPID
lomitapide mesylate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10122-420 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOMITAPIDE MESYLATE (UNII: X4S83CP54E) (LOMITAPIDE - UNII:82KUB0583F) LOMITAPIDE 20 mg Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code A733;20mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10122-420-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 01/28/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203858 01/03/2013 JUXTAPID
lomitapide mesylate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10122-430 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOMITAPIDE MESYLATE (UNII: X4S83CP54E) (LOMITAPIDE - UNII:82KUB0583F) LOMITAPIDE 30 mg Product Characteristics Color yellow, orange (Swedish Orange) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code A733;30mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10122-430-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 06/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203858 01/03/2013 Labeler - Chiesi USA, Inc. (088084228)
Trademark Results [Juxtapid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 JUXTAPID 97193314 not registered Live/Pending |
Amryt Pharmaceuticals Inc. 2021-12-28 |
 JUXTAPID 97193306 not registered Live/Pending |
Amryt Pharmaceuticals Inc. 2021-12-28 |
 JUXTAPID 85981114 4512713 Live/Registered |
Aegerion Pharmaceuticals, Inc. 2013-02-22 |
 JUXTAPID 85980934 4508190 Live/Registered |
Aegerion Pharmaceuticals, Inc. 2012-12-14 |
 JUXTAPID 85857475 not registered Dead/Abandoned |
Aegerion Pharmaceuticals, Inc. 2013-02-22 |
 JUXTAPID 85802929 not registered Dead/Abandoned |
Aegerion Pharmaceuticals, Inc. 2012-12-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.