ITCHY EYE DROPS- ketotifen fumarate solution/ drops
Itchy Eye Drops by
Drug Labeling and Warnings
Itchy Eye Drops by is a Otc medication manufactured, distributed, or labeled by Good Sense, Akorn, Inc. , Akorn, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
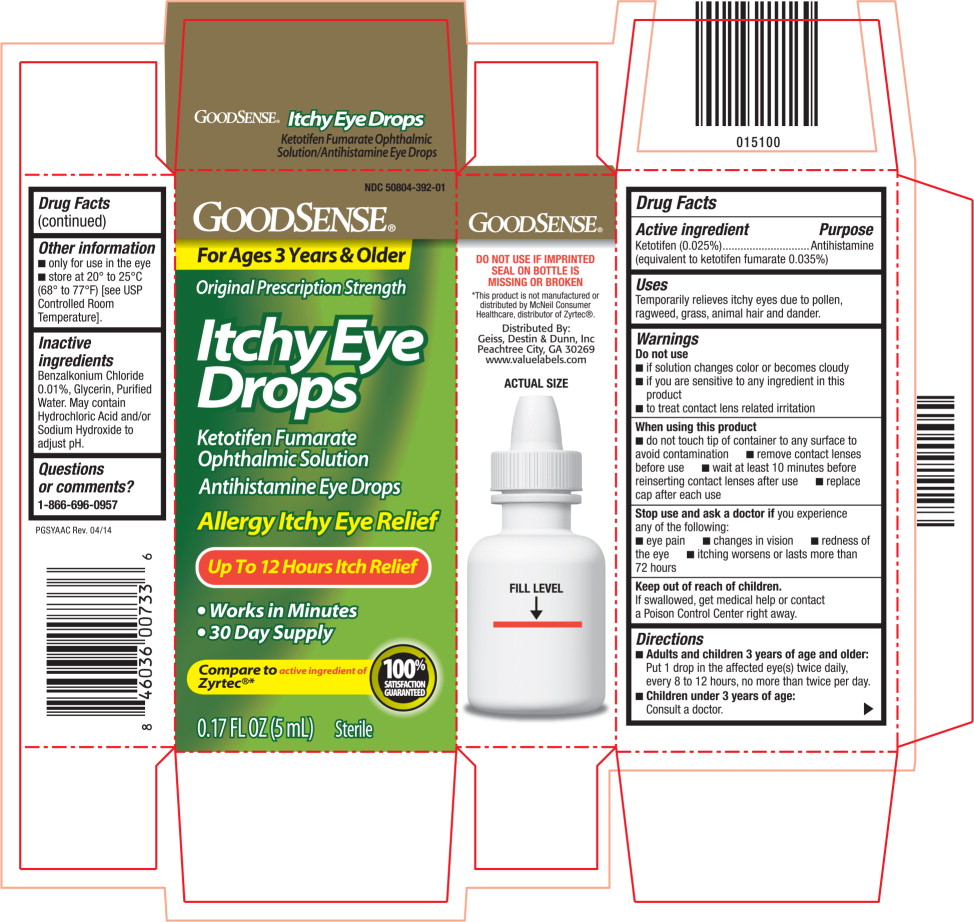
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 50804-392-01
GOODSENSE® Logo
Itchy Eye Drops
Ketotifen Fumarate
Ophthalmic Solution
Antihistamine Eye Drops
0.17 FL OZ (5 mL) Sterile
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 50804-392-01
GOODSENSE® Logo
For Ages 3 Years & Older
Original Prescription Strength
Itchy Eye
Drops
Ketotifen Fumarate
Ophthalmic Solution
Antihistamine Eye Drops
Allergy Itchy Eye Relief
Up To 12 Hours Itch Relief
● Works in Minutes
● 30 Day Supply
Compare to active ingredient of 100%
Zyrtec®* SATISFACTION
GUARANTEED
0.17 FL OZ (5 mL) Sterile
-
INGREDIENTS AND APPEARANCE
ITCHY EYE DROPS
ketotifen fumarate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50804-392 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ketotifen Fumarate (UNII: HBD503WORO) (Ketotifen - UNII:X49220T18G) Ketotifen 0.35 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Glycerin (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50804-392-01 1 in 1 CARTON 1 5 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077958 06/20/2014 Labeler - Good Sense (076059836) Registrant - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 603980319 MANUFACTURE(50804-392) , ANALYSIS(50804-392) , STERILIZE(50804-392) , PACK(50804-392) , LABEL(50804-392)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.