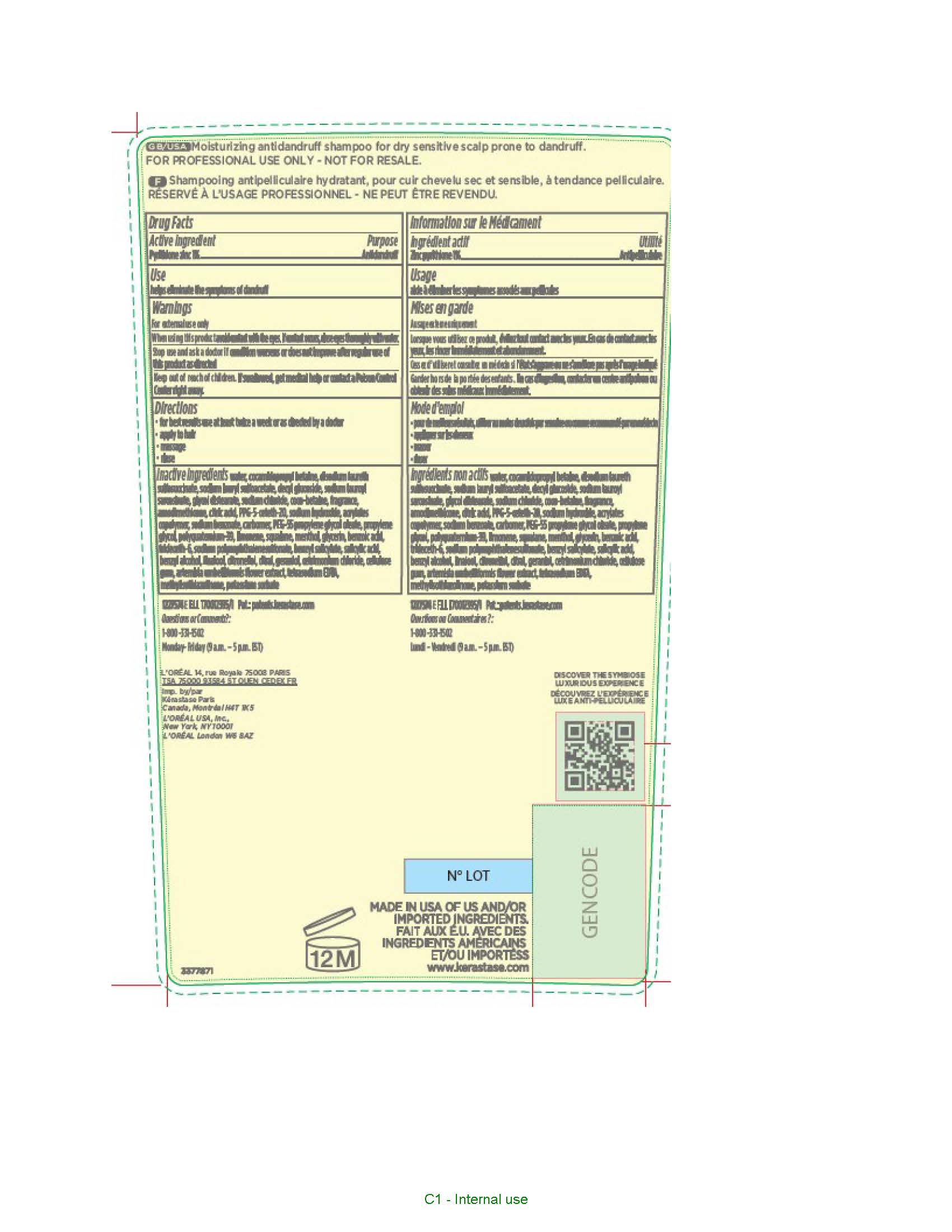
Kerastase Paris Symbiose Antidandruff by L'Oreal USA Products Inc / L'OREAL USA, INC Drug Facts
Kerastase Paris Symbiose Antidandruff by
Drug Labeling and Warnings
Kerastase Paris Symbiose Antidandruff by is a Otc medication manufactured, distributed, or labeled by L'Oreal USA Products Inc, L'OREAL USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KERASTASE PARIS SYMBIOSE ANTIDANDRUFF- pyrithione zinc shampoo
L'Oreal USA Products Inc
----------
Drug Facts
When using this product
avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
condition worsens or does not improve after regular use of this product as directed
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- for best results use at least twice a week or as directed by a doctor
- apply to hair
- massage
- rinse
Inactive ingredients
water, cocamidopropyl betaine, disodium laureth sulfosuccinate, sodium lauryl sulfoacetate, decyl glucoside, sodium lauroyl sarcosinate, glycol distearate, sodium chloride, coco-betaine, fragrance, amodimethicone, citric acid, PPG-5-ceteth-20, sodium hydroxide, acrylates copolymer, sodium benzoate, carbomer, PEG-55 propylene glycol oleate, propylene glycol, polyquaternium-39, limonene, squalane, menthol, glycerin, benzoic acid, trideceth-6, sodium polynaphthalenesulfonate, benzyl salicylate, salicylic acid, benzyl alcohol, linalool, citronellol, citral, geraniol, cetrimonium chloride, cellulose gum, artemisia umbelliformis flower extract, tetrasodium EDTA, methylisothiazolinone, potassium sorbate
| KERASTASE PARIS SYMBIOSE ANTIDANDRUFF
pyrithione zinc shampoo |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L'Oreal USA Products Inc (002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| L'OREAL USA, INC | 960317444 | manufacture(49967-871) , pack(49967-871) | |