LINCOMYCIN- lincomycin hydrochloride injection, solution
Lincomycin by
Drug Labeling and Warnings
Lincomycin by is a Prescription medication manufactured, distributed, or labeled by XGen Pharmaceuticals DJB, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Lincomycin and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
Because lincomycin therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate, as described in the INDICATIONS AND USAGE section. It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections.
C.diffficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
DESCRIPTION
Lincomycin Injection is a sterile solution which contains lincomycin hydrochloride which is the monohydrated salt of lincomycin, a substance produced by the growth of a member of the lincolnensis group of Streptomyces lincolnensis (Fam. Streptomycetaceae). The chemical name for lincomycin hydrochloride is Methyl 6,8-dideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrolidinecarboxamido)-1-thio-D-erythro-α-D-galacto-octopyranoside monohydrochloride monohydrate. The molecular formula of lincomycin hydrochloride is C18H34N2O6S.HCl.H2O and the molecular weight is 461.01.
The structural formula is represented below:
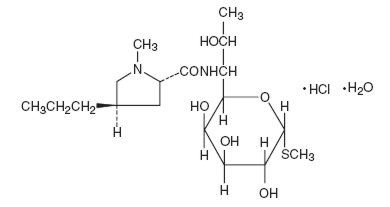
Chemical Structure
Lincomycin hydrochloride is a white or practically white, crystalline powder and is odorless or has a faint odor. Its solutions are acid and are dextrorotatory. Lincomycin hydrochloride is freely soluble in water; soluble in dimethylformamide and very slightly soluble in acetone.
Each mL contains Lincomycin hydrochloride equivalent to 300 mg lincomycin. Also contains 9.45 mg benzyl alcohol added as a preservative.
-
CLINICAL PHARMACOLOGY
Intramuscular administration of a single dose of 600 mg of lincomycin produces average peak serum concentrations of 11.6 µg/mL at 60 minutes and maintains therapeutic concentrations for 17 to 20 hours for most susceptible gram-positive organisms. Urinary excretion after this dose ranges from 1.8 to 24.8 percent (mean: 17.3 percent).
A two hour intravenous infusion of 600 mg of lincomycin achieves average peak serum concentrations of 15.9 µg/mL and yields therapeutic concentrations for 14 hours for most susceptible gram-positive organisms. Urinary excretion ranges from 4.9 to 30.3 percent (mean: 13.8 percent).
The biological half-life after intramuscular or intravenous administration is 5.4 ± 1.0 hours. The serum half-life of lincomycin may be prolonged in patients with severe impairment of renal function compared to patients with normal renal function. In patients with abnormal hepatic function, serum half-life may be twofold longer than in patients with normal hepatic function. Hemodialysis and peritoneal dialysis are not effective in removing lincomycin from the serum.
Tissue level concentrations indicate that bile is an important route of excretion. Significant concentrations have been demonstrated in the majority of body tissues. Although lincomycin appears to diffuse into cerebrospinal fluid (CSF), concentrations of lincomycin in the CSF appear inadequate for the treatment of meningitis.
Microbiology
Mechanism of Action
Lincomycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the bacterial ribosome. Lincomycin is predominantly bacteriostatic in vitro.
Resistance
Cross resistance has been demonstrated between clindamycin and lincomycin. Resistance is most often due to methylation of specific nucleotides in the 23S RNA of the 50S ribosomal subunit, which can determine cross resistance to macrolides and streptogramins B (MLS phenotype). Macrolide-resistant isolates of these organisms should be tested for inducible resistance to lincomycin/clindamycin using the D-zone test or other appropriate method.
Antimicrobial ActivityLincomycin has been shown to be active against most strains of the following organisms both in vitro and in clinical infections: (see INDICATIONS AND USAGE).
Staphylococcus aureus
Streptococcus pneumoniaeThe following in vitro data are available; but their clinical significance is unknown.
Lincomycin has been shown to be active in vitro against the following microorganisms; however, the safety and efficacy of Lincomycin in treating clinical infections due to these organisms have not been established in adequate and well controlled trials.
Gram-positive bacteria::
Corynebacterium diphtheriae
Streptococcus pyogenes
Viridans group streptococciAnaerobic bacteria:
Clostridium tetani
Clostridium perfringensSusceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
INDICATIONS AND USAGE
Lincomycin Injection, USP is indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of CDAD, as described in the BOXED WARNING before selecting lincomycin the physician should consider the nature of the infection and the suitability of other alternatives.
Indicated surgical procedures should be performed in conjunction with antibacterial therapy.
The drug may be administered concomitantly with other antimicrobial agents when indicated.
Lincomycin is not indicated in the treatment of minor bacterial infections or viral infections.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Lincomycin and other antibacterial drugs, Lincomycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
See BOXED WARNING.
Clostridium difficile associated diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Lincomycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Hypersensitivity
Severe hypersensitivity reactions, including anaphylactic reactions and severe cutaneous adverse reactions (SCAR) such as StevensJohnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and erythema multiforme (EM) have been reported in patients receiving Lincomycin Injection, USP therapy. If an anaphylactic reaction or severe skin reaction occurs, Lincomycin Injection, USP should be discontinued and appropriate therapy should be initiated. (See ADVERSE REACTIONS)
Benzyl Alcohol Toxicity in Pediatric Patients (Gasping Syndrome)
This product contains benzyl alcohol as a preservative.
The preservative benzyl alcohol has been associated with serious adverse events, including the "gasping syndrome", and death in pediatric patients. Although normal therapeutic doses of this product ordinarily deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the "gasping syndrome", the minimum amount of benzyl alcohol at which toxicity may occur is not known. The risk of benzyl alcohol toxicity depends on the quantity administered and the liver and kidney's capacity to detoxify the chemical. Premature and low-birth weight infants may be more likely to develop toxicity.
Use in Meningitis — Although lincomycin appears to diffuse into cerebrospinal fluid, levels of lincomycin in the CSF may be inadequate for the treatment of meningitis.
-
PRECAUTIONS
General
Review of experience to date suggests that a subgroup of older patients with associated severe illness may tolerate diarrhea less well. When Lincomycin is indicated in these patients, they should be carefully monitored for change in bowel frequency.
Lincomycin should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Lincomycin should be used with caution in patients with a history of asthma or significant allergies.
Certain infections may require incision and drainage or other indicated surgical procedures in addition to antibacterial therapy.
The use of Lincomycin may result in overgrowth of nonsusceptible organisms— particularly yeasts. Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation. When patients with pre-existing monilial infections require therapy with Lincomycin, concomitant antimonilial treatment should be given.
The serum half-life of lincomycin may be prolonged in patients with severe impairment of renal function compared to patients with normal renal function. In patients with abnormal hepatic function, serum half-life may be twofold longer than in patients with normal hepatic function.
Patients with severe impairment of renal function and/or abnormal hepatic function should be dosed with caution and serum lincomycin levels monitored during high-dose therapy. (See DOSAGE AND ADMINISTRATION Section.)
Lincomycin should not be injected intravenously undiluted as a bolus, but should be infused over at least 60 minutes as directed in the DOSAGE AND ADMINISTRATION Section.
Prescribing Lincomycin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs including Lincomycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Lincomycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Lincomycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterial which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterial, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible
Laboratory Tests
During prolonged therapy with Lincomycin, periodic liver and kidney function tests and blood counts should be performed.
Drug Interactions
Lincomycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuro-muscular blocking
agents. Therefore, it should be used in caution in patients receiving such agents.Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of lincomycin has not been evaluated.
Lincomycin was not found to be mutagenic in the Ames Salmonella reversion assay or the V79 Chinese hamster lung cells at the HGPRT locus. It did not induce DNA strand breaks in V79 Chinese hamster lung cells as measured by alkaline elution or chromosomal abnormalities in cultured human lymphocytes. In vivo, lincomycin was negative in both the rat and mouse micronucleus assays and it did not induce sex-linked recessive lethal mutations in the offspring of male Drosophila. However, lincomycin did cause unscheduled DNA syntheses in freshly isolated rat hepatocytes.
Impairment of fertility was not observed in male or female rats given oral 300 mg/kg doses of lincomycin (0.36 times the highest recommended human dose based on mg/m2).
Pregnancy
There are no adequate and well-controlled studies in pregnant women. Lincomycin Injection , USP Sterile Solution contains benzyl alcohol as a preservative. Benzyl alcohol can cross the placenta. See WARNINGS. Lincomycin for Injection, USP should be used during pregnancy only if clearly needed.
Teratogenic Effects
In a study with 60 pregnant women, cord serum concentrations were approximately 25% of the maternal serum concentrations, indicating that lincomycin crosses the placenta, and no substantial accumulation occurred in the amniotic fluid. Experience with 345 obstetrical patients receiving Lincomycin Injection, USP revealed no ill effects related to pregnancy.
There was no evidence of teratogenicity when lincomycin was administered in diet to pregnant Sprague Dawley rats during the period of major organogenesis at doses up to 5000 mg/kg (approximately 6 times the maximum recommended human dose [MRHD], respectively, based on body surface area comparison).
Nonteratogenic Effects
Reproduction studies performed in rats administered oral lincomycin in diet for 2 weeks prior to mating, throughout pregnancy and lactation, revealed no adverse effects on survival of offspring from birth to weaning at doses up to 1000 mg/kg (1.2 times the MRHD based on body surface area comparison) up to 2 generations.
Nursing Mothers
Lincomycin has been reported to appear in human milk in concentrations of 0.5 to 2.4 mcg/mL. Because of the potential for serious adverse reactions in nursing infants from Lincomycin, a decision should be made whether to discontinue nursing, or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Lincomycin Injection, USP contains benzyl alcohol as a preservative. Benzyl alcohol has been associated with a fatal "Gasping Syndrome" in premature infants. See WARNINGS. Safety and effectiveness in pediatric patients below the age of one month have not been established. (See DOSAGE AND ADMINISTRATION Section.)
-
ADVERSE REACTIONS
The following reactions have been reported with the use of lincomycin:
Gastrointestinal disorders
Diarrhea, nausea, vomiting, glossitis, stomatitis, abdominal pain, abdominal discomfort1 , anal pruritus
1 Event has been reported with intravenous injection.
Skin and subcutaneous tissue disorders
Toxic epidermal necrolysis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis,dermatitis bullous, dermatitis exfoliative, erythema multiforme (see WARNINGS), rash, urticaria, pruritus
Infections and infestations
Vaginal infection, pseudomembranous colitis, Clostridium difficile colitis (see Vaginal infection, pseudomembranous colitis, Clostridium diffi cile colitis (see WARNINGS))
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
If significant diarrhea occurs during therapy, this antibacterial should be discontinued. (See BOXED WARNING)
INTRAMUSCULAR
Adults
Serious infections—600 mg (2 mL) intramuscularly every 24 hours. More severe infections—600 mg (2 mL) intramuscularly every 12 hours or more often.
Pediatric patients over 1 month of age: Serious infections—one intramuscular injection of 10 mg/kg (5 mg/lb) every 24 hours. More severe infections—one intramuscular injection of 10 mg/kg (5 mg/lb) every 12 hours or more often.
INTRAVENOUS
Adults:
The intravenous dose will be determined by the severity of the infection. For serious infections doses of 600 mg of lincomycin (2 mL of Lincomycin Injection) to 1 gram are given every 8 to 12 hours. For more severe infections these doses may have to be increased. In life-threatening situations, daily intravenous doses of as much as 8 grams have been given. Intravenous doses are given on the basis of 1 gram of lincomycin diluted in not less than 100 mL of appropriate solution (see PHYSICAL COMPATIBILITIES) and infused over a period of not less than one hour.
Dose Vol. Diluent Time
600 mg 100 mL 1 hr
1 gram 100 mL 1 hr
2 grams 200 mL 2 hr
3 grams 300 mL 3 hr
4 grams 400 mL 4 hr
These doses may be repeated as often as required to the limit of the maximum recommended daily dose of 8 grams of lincomycin. Pediatric patients over 1 month of age 10 to 20 mg/kg/day (5 to 10 mg/lb/day) depending on the severity of the infection may be infused in divided doses as described above for adults.
NOTE: Severe cardiopulmonary reactions have occurred when this drug has been given at greater than the recommended concentration and rate.
SUBCONJUNCTIVAL INJECTION
0.25 mL (75 mg) injected subconjunctivally will result in ocular fluid levels of antibacterial (lasting for at least 5 hours) with MICs sufficient for most susceptible pathogens.
Patients with diminished renal function
When therapy with Lincomycin is required in individuals with severe impairment of renal function, an appropriate dose is 25 to 30% of that recommended for patients with normally functioning kidneys.
-
HOW SUPPLIED
Lincomycin Injection, USP is available in the following strength and package sizes:
300 mg/mL
2 mL Vials — NDC: 39822-0350-1 Packaged as 10 vials per carton NDC: 39822-0350-2
10 mL Vials — NDC: 39822-0353-5 Packaged as 10 vials per carton NDC: 39822-0353-6
Each mL of Lincomycin Injection, USP contains lincomycin hydrochloride equivalent to lincomycin 300 mg; also benzyl alcohol, 9.45 mg added as preservative.
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
-
ANIMAL PHARMACOLOGY
In vivo experimental animal studies demonstrated the effectiveness of LINCOCIN preparations (lincomycin) in protecting animals infected with Streptococcus viridans,β-hemolytic Streptococcus, Staphylococcus aureus, Diplococcus pneumoniae and Leptospira pomona. It was ineffective in Klebsiella, Pasteurella, Pseudomonas, Salmonella and Shigella infections.
-
PHYSICAL COMPATIBILITIES
Physically compatible for 24 hours at room temperature unless otherwise indicated.
Infusion Solutions
5% Dextrose Injection
10% Dextrose Injection
5% Dextrose and 0.9% Sodium Chloride Injection
10% Dextrose and 0.9% Sodium Chloride Injection
Ringer's Injection1/6 M Sodium Lactate Injection
Travert 10%-Electrolyte No. 1
Dextran in Saline 6% w/vVitamins in Infusion Solutions
B-Complex
B-Complex with Ascorbic AcidAntibacterial in Infusion Solutions
Penicillin G Sodium (Satisfactory for 4 hours)
Cephalothin
Tetracycline HCl
Cephaloridine
Colistimethate (Satisfactory for 4 hours)
Ampicillin
Methicillin
Chloramphenicol
Polymyxin B SulfatePhysically Incompatible with:
Novobiocin
KanamycinIT SHOULD BE EMPHASIZED THAT THE COMPATIBLE AND INCOMPATIBLE DETERMINATIONS ARE PHYSICAL OBSERVATIONS ONLY, NOT CHEMICAL DETERMINATIONS. ADEQUATE CLINICAL EVALUATION OF THE SAFETY AND EFFICACY OF THESE COMBINATIONS HAS NOT BEEN PERFORMED.
-
PRINCIPAL DISPLAY PANEL - 1-2 mL Vial Label
X-GEN Pharmaceuticals, Inc.
NDC: 39822-0350-1
lincomycin injection, USP
300 mg/mL
1-2 mL Vial
Rx only
2 ml label
-
PRINCIPAL DISPLAY PANEL - 1-10 mL Vial Label
X-GEN Pharmaceuticals, Inc.
NDC: 39822-0353-5
lincomycin injection, USP
300 mg/mL
1-10 mL Vial
Rx only
linco-10ml-vial
-
INGREDIENTS AND APPEARANCE
LINCOMYCIN
lincomycin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 39822-0350 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCONJUNCTIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 9.45 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 39822-0350-2 10 in 1 CARTON 07/05/2015 1 NDC: 39822-0350-1 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201746 07/05/2015 LINCOMYCIN
lincomycin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 39822-0353 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCONJUNCTIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 9.45 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 39822-0353-6 10 mL in 1 VIAL; Type 0: Not a Combination Product 07/05/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201746 07/05/2015 Labeler - X-GEN Pharmaceuticals, Inc. (790169531)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.