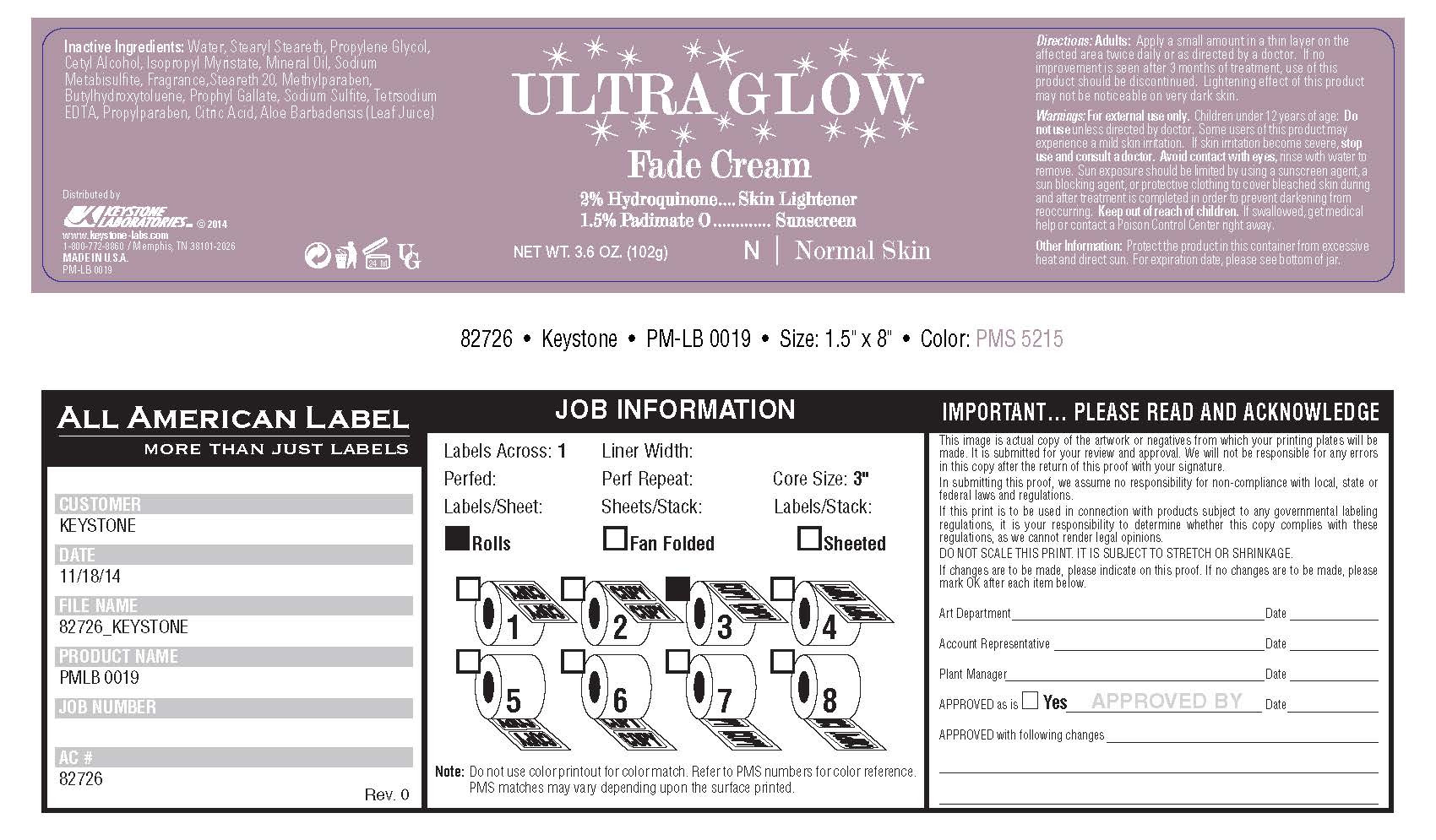
ULTRA GLOW FADE- hydroquinone cream
Ultra Glow Fade by
Drug Labeling and Warnings
Ultra Glow Fade by is a Otc medication manufactured, distributed, or labeled by Keystone Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
For external use only. Children under 12 years of age: Do not use unless directed by a doctor. Some users of this product may experience a mild skin irritation. If skin irritation becomes severe, stop use and consult a doctor. Avoid contact with eyes, rinse with water to remove. Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin during and after treatment is completed in order to prevent darkening from reoccurring. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive Ingredients:
water, stearyl stearate, propylene glycol, cetyl alcohol, isopropyl myristate, mineral oil, sodium metabisulfite, fragrance, steareth 20, methylparaben, butylhydoxytoluene, prophyl gallate, sodium sulfite, sodium sulfite, tetrasodium EDTA, propylparaben, citric acid, aloe barbadensis (Leaf Juice)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ULTRA GLOW FADE
hydroquinone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58318-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 2 g in 102 g PADIMATE O (UNII: Z11006CMUZ) (PADIMATE O - UNII:Z11006CMUZ) PADIMATE O 1.5 g in 102 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CETYL ALCOHOL (UNII: 936JST6JCN) SODIUM DITHIONATE (UNII: RPF7Z41GAW) STEARYL STEARATE (UNII: 5WX2EGD0DK) STEARETH-20 (UNII: L0Q8IK9E08) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PROPYL GALLATE (UNII: 8D4SNN7V92) SODIUM METABISULFITE (UNII: 4VON5FNS3C) EDETATE DISODIUM (UNII: 7FLD91C86K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYL ALCOHOL (UNII: Y4S76JWI15) SODIUM SULFITE (UNII: VTK01UQK3G) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58318-008-01 102 g in 1 JAR; Type 0: Not a Combination Product 01/04/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part358A 01/04/2018 Labeler - Keystone Laboratories (007017429) Establishment Name Address ID/FEI Business Operations Keystone Laboratories 007017429 manufacture(58318-008)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.