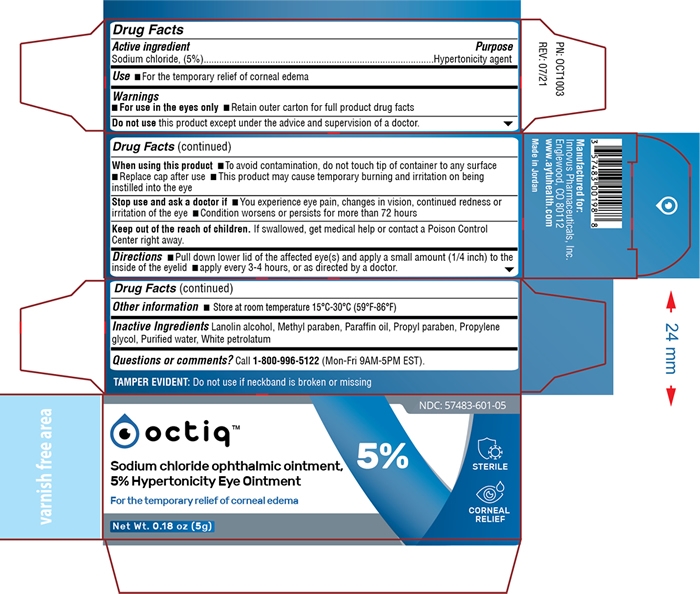
Octiq Sodium Chloride Eye Ointment
Octiq by
Drug Labeling and Warnings
Octiq by is a Otc medication manufactured, distributed, or labeled by Innovus Pharmaceuticals, Inc., AMMAN PHARMACEUTICAL INDUSTRIES. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OCTIQ- sodium chloride ointment
Innovus Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Octiq Sodium Chloride Eye Ointment
When using this product
- To avoid contamination, do not touch tip of container to any surface
- Replace cap after use
- This product may cause temporary burning and irritation on being instilled into the eye
Stop use and ask a doctor if
- You experience eye pain, changes in vision, continued redness or irritation of the eye
- Condition worsens or persists for more than 72 hours
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- pull down lower lid of the affected eye(s) and apply a small amount (1/4 inch) to the inside of the eyelid
- apply every 3 - 4 hours, or as directed by a doctor.
| OCTIQ
sodium chloride ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Innovus Pharmaceuticals, Inc. (962507187) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMMAN PHARMACEUTICAL INDUSTRIES | 534677849 | manufacture(57483-601) | |
Revised: 7/2023
Document Id: 875ca176-e8b4-45ae-9d27-e329b0f860c6
Set id: 61fffceb-7f35-45a7-a7b7-c3b31c240fbc
Version: 3
Effective Time: 20230712
Trademark Results [Octiq]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OCTIQ 97470919 not registered Live/Pending |
Innovus Pharmaceuticals, Inc. 2022-06-22 |
 OCTIQ 97116304 not registered Live/Pending |
Innovus Pharmaceuticals, Inc. 2021-11-09 |
 OCTIQ 88179843 not registered Live/Pending |
Innovus Pharmaceuticals, Inc. 2018-11-02 |
 OCTIQ 77873209 not registered Dead/Abandoned |
Pink OTC Markets Inc. 2009-11-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.