EOVIST- gadoxetate disodium injection, solution
EOVIST by
Drug Labeling and Warnings
EOVIST by is a Prescription medication manufactured, distributed, or labeled by Bayer HealthCare Pharmaceuticals Inc., Bayer AG, QUALITY PACKAGING SPECIALISTS INTERNATIONAL LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EOVIST® safely and effectively. See full prescribing information for EOVIST Injection.
EOVIST (gadoxetate disodium) injection, for intravenous use
Initial U.S. Approval: 2008WARNING: NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
See full prescribing information for complete boxed warning.
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities.
-
The risk for NSF appears highest among patients with:
- o Chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- o Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function.
For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing (5.1).
RECENT MAJOR CHANGES
Dosage and Administration, Drug Handling and Administration (2.2) 5/2019
INDICATIONS AND USAGE
EOVIST is a gadolinium-based contrast agent indicated for use in magnetic resonance imaging (MRI) of the liver to detect and characterize lesions in patients with known or suspected focal liver disease (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 181.43 mg/mL in single-dose containers (vials) (3)
CONTRAINDICATIONS
History of severe hypersensitivity reaction to EOVIST (4)
WARNINGS AND PRECAUTIONS
- Nephrogenic Systemic Fibrosis has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeated dosing appears to increase the risk (5.1)
- Hypersensitivity: anaphylactoid/hypersensitivity reactions with cardiovascular, respiratory and cutaneous manifestations, ranging from mild to severe reactions including shock can occur. Monitor patients closely for need of emergency cardiorespiratory support (5.2)
- Gadolinium is retained for months or years in brain, bone, and other organs. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 0.5%) are nausea, headache, feeling hot, dizziness, and back pain (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-84-BAYER (1-888-842-2937) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Use only if imaging is essential during pregnancy and cannot be delayed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2015
-
The risk for NSF appears highest among patients with:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Drug Handling and Administration
2.3 Imaging
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Nephrogenic Systemic Fibrosis (NSF)
5.2 Hypersensitivity Reactions
5.3 Gadolinium Retention
5.4 Acute Kidney Injury
5.5 Extravasation and Injection Site Reactions
5.6 Interference with Laboratory Tests
5.7 Interference with Visualization of Liver Lesions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
-
The risk for NSF appears highest among patients with:
- o Chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- o Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
For patients at highest risk for NSF, do not exceed the recommended EOVIST dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration [see Warnings and Precautions (5.1)].
-
The risk for NSF appears highest among patients with:
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of EOVIST is 0.1 mL/kg body weight (0.025 mmol/kg body weight).
2.2 Drug Handling and Administration
- Use sterile technique when preparing and administering EOVIST
- Visually inspect EOVIST, supplied in a single-dose container (vial), for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored or if particulate matter is present
- Use EOVIST immediately after obtaining appropriate dose from vial. The rubber stopper should never be pierced more than once. Discard any unused portion of an EOVIST vial
- Administer EOVIST undiluted as an intravenous bolus injection at a flow rate of approximately 2 mL/second
- Do not mix EOVIST with other medications and do not administer EOVIST in the same intravenous line simultaneously with other medications
- Flush the intravenous cannula with a normal saline solution after EOVIST injection
- Imaging can commence immediately following EOVIST administration
2.3 Imaging
- Liver lesions are detected and characterized with pre-contrast MRI and EOVIST MRI obtained during dynamic and hepatocyte imaging phases. Perform a pre-contrast MRI, inject EOVIST and begin dynamic imaging approximately 15–25 seconds after completion of the injection. Dynamic imaging consists of the arterial, the porto-venous (approximately 60 seconds post-injection), and the blood equilibrium (approximately 120 seconds) phases.
- Begin the hepatocyte imaging phase approximately 20 minutes post-injection. Hepatocyte phase imaging may be performed up to 120 minutes post-injection.
- Elevated intrinsic levels of bilirubin (>3 mg/dL) or ferritin can reduce the hepatic contrast effect of EOVIST. Perform MR imaging no later than 60 minutes following EOVIST administration to patients with these laboratory abnormalities, including patients who have elevated ferritin levels due to hemodialysis [see Warnings and Precautions (5.6) and Use in Specific Populations (8.6, 8.7)].
- Lesions with no or minimal hepatocyte function (cysts, metastases, and the majority of hepatocellular carcinomas) generally will not accumulate EOVIST. Well-differentiated hepatocellular carcinoma may contain functioning hepatocytes and can show some enhancement in the hepatocyte imaging phase. Additional clinical information is therefore needed to support a diagnosis of hepatocellular carcinoma.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Nephrogenic Systemic Fibrosis (NSF)
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR < 30 mL/min/1.73m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30 to 59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60 to 89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following EOVIST administration to Bayer HealthCare (1-888-842-2937) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch).
Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (for example, age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administrated to a patient. For patients at highest risk for NSF, do not exceed the recommended EOVIST dose and allow a sufficient period of time for elimination of the drug prior to any re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. The usefulness of hemodialysis in the prevention of NSF is unknown.
5.2 Hypersensitivity Reactions
Anaphylactic and other hypersensitivity reactions with cardiovascular, respiratory and cutaneous manifestations, ranging from mild to severe, including shock have uncommonly occurred following EOVIST administration [see Adverse Reactions (6)].
- Before EOVIST administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to EOVIST.
- Administer EOVIST only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, including personnel trained in resuscitation.
Most hypersensitivity reactions to EOVIST have occurred within half an hour after administration. Delayed reactions can occur up to several days after EOVIST administration. Observe patients for signs and symptoms of hypersensitivity reactions during and following EOVIST administration.
5.3 Gadolinium Retention
Gadolinium is retained for months or years in several organs. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (for example, brain, skin, kidney, liver, and spleen). The duration of retention also varies by tissue and is longest in bone. Linear GBCAs cause more retention than macrocyclic GBCAs. At equivalent doses, gadolinium retention varies among the linear agents with Omniscan (gadodiamide) and Optimark (gadoversetamide) causing greater retention than other linear agents [Eovist (gadoxetate disodium), Magnevist (gadopentetate dimeglumine),MultiHance (gadobenate dimeglumine)]. Retention is lowest and similar among the macrocyclic GBCAs [Dotarem (gadoterate meglumine), Gadavist (gadobutrol), ProHance (gadoteridol)].
Consequences of gadolinium retention in the brain have not been established. Pathologic and clinical consequences of GBCA administration and retention in skin and other organs have been established in patients with impaired renal function [see Warnings and Precautions (5.1)]. There are rare reports of pathologic skin changes in patients with normal renal function. Adverse events involving multiple organ systems have been reported in patients with normal renal function without an established causal link to gadolinium retention [see Adverse Reactions (6.2)].
While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Consider the retention characteristics of the agent when choosing a GBCA for these patients. Minimize repetitive GBCA imaging studies particularly closely spaced studies, when possible.
5.4 Acute Kidney Injury
In patients with chronic renal impairment, acute kidney injury sometimes requiring dialysis has been observed with the use of some GBCAs. The risk of acute kidney injury might be lower with EOVIST due to its dual excretory pathways. Do not exceed the recommended dose; the risk of acute kidney injury may increase with higher than recommended doses.
5.5 Extravasation and Injection Site Reactions
Ensure catheter and venous patency before the injection of EOVIST. Extravasation into tissues during EOVIST administration may result in local tissue reactions. Strictly avoid intramuscular administration of EOVIST because it may cause myocyte necrosis and inflammation [see Nonclinical Toxicology (13.2)].
5.6 Interference with Laboratory Tests
Serum iron determination using complexometric methods (for example, ferrocene complexation method) may result in falsely high or low values for up to 24 hours after the examination with EOVIST because of the caloxetate trisodium excipients [see Adverse Reactions (6.1)].
5.7 Interference with Visualization of Liver Lesions
Severe renal or hepatic failure may impair EOVIST imaging performance. In patients with end-stage renal failure, hepatic contrast was markedly reduced and was attributed to elevated serum ferritin levels. In patients with abnormally high (>3 mg/dL) serum bilirubin, reduced hepatic contrast was observed. If EOVIST is used in these patients, complete MRI no later than 60 minutes after EOVIST administration and use a paired non-contrast and contrast MRI set for diagnosis.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Nephrogenic systemic fibrosis (NSF) [see Boxed Warning and Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Contraindications (4) and Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The adverse reactions described in this section reflect EOVIST exposure in 1,989 subjects with the majority (1,581 subjects) receiving the recommended dose. Overall, 59% of the subjects were men and the ethnic distribution was 64% Caucasian, 22% Asian, 3% Hispanic, 2% Black, and 0.5% of subjects consisted of other ethnic groups. The average age was 57 years (age range from 19 to 84 years).
Overall, 4% of subjects reported one or more adverse reactions following EOVIST administration. The most frequent (≥ 0.5%) adverse reactions associated with the use of EOVIST were nausea, headache, feeling hot, dizziness, and back pain. Adverse reactions were predominantly of mild to moderate severity.
Table 1 lists adverse reactions that occurred in ≥ 0.1% of subjects treated with EOVIST.
Table 1 Adverse Reactions Reaction
Rate (%)
n = 1581Nausea
1.1
Headache
1.1
Feeling hot
0.8
Dizziness
0.6
Back pain
0.6
Vomiting
0.4
Blood pressure increased
0.4
Injection site reactions (pain, burning, coldness, extravasation, irritation)
0.4
Dysgeusia
0.4
Paresthesia
0.3
Flushing
0.3
Parosmia
0.3
Pruritus (generalized, eye)
0.3
Rash
0.3
Respiratory disorders (dyspnea, respiratory distress)
0.2
Fatigue
0.2
Chest pain
0.1
Vertigo
0.1
Dry mouth
0.1
Chills
0.1
Feeling abnormal
0.1
Adverse reactions that occurred with a frequency of < 0.1% in subjects who received EOVIST include: tremor, akathisia, bundle branch block, palpitation, oral discomfort, salivary hypersecretion, maculopapular rash, hyperhidrosis, discomfort, and malaise.
Elevation of serum iron values and serum bilirubin laboratory values were reported in less than 1% of patients after administration of EOVIST. The values did not exceed more than 3 times the baseline values and returned to baseline within 1 to 4 days.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported during the postmarketing use of EOVIST. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions (anaphylactic shock, hypotension, pharyngolaryngeal edema, urticaria, face edema, rhinitis, conjunctivitis, abdominal pain, hypoesthesia, sneezing, cough and pallor) [see Warnings and Precautions (5.2)]
- Tachycardia
- Restlessness
- General Disorders and Administration Site Conditions: Adverse events with variable onset and duration have been reported after GBCA administration [see Warnings and Precautions (5.3)]. These include fatigue, asthenia, pain syndromes, and heterogeneous clusters of symptoms in the neurological, cutaneous, and musculoskeletal systems.
- Skin: Gadolinium associated plaques
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
GBCAs have been shown to cross the human placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes are limited and inconclusive (see Data). In animal
reproduction studies, no teratogenicity was observed with repeated daily intravenous administration of gadoxetate disodium to rats during organogenesis at doses up to 32 times the recommended single human dose; however, an increase in preimplantation loss was noted at doses 3.2 times the single human dose. Post implantation loss was observed with repeated daily intravenous administration of gadoxetate disodium to rabbits on gestation days 6 through 18 at doses 26 times the recommended single human dose (see Data). Because of the potential risks of gadolinium to the fetus, use EOVIST only if imaging is essential during pregnancy and cannot be delayed.
The background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Human Data
Contrast enhancement is visualized in the placenta and fetal tissues after maternal GBCA administration.
Cohort studies and case reports on exposure to GBCAs during pregnancy have not reported a clear association between GBCAs and adverse effects in the exposed neonates. However, a retrospective cohort study, comparing pregnant women who had a GBCA MRI to pregnant women who did not have an MRI, reported a higher occurrence of stillbirths and neonatal deaths in the group receiving GBCA MRI. Limitations of this study include a lack of comparison with non-contrast MRI and lack of information about the maternal indication for MRI. Overall, these data preclude a reliable evaluation of the potential risk of adverse fetal outcomes with the use of GBCAs in pregnancy.
Animal Data
Gadolinium Retention
GBCAs administered to pregnant non-human primates (0.1 mmol/kg on gestational days 85 and 135) result in measurable gadolinium concentration in the offspring in bone, brain, skin, kidney, and spleen for at least 7 months. GBCAs administered to pregnant mice (2 mmol/kg daily on gestational days 16 through 19) result in measurable gadolinium concentrations in the pups in bone, brain, kidney, liver, blood, muscle, and spleen at one month postnatal age.
Reproductive Toxicology
Animal reproductive and developmental toxicity studies were done in rats and rabbits. Gadoxetate disodium was not teratogenic when given intravenously during organogenesis to pregnant rats at doses up to 32 times the recommended single human dose (mmol/m2 basis). However, an increase in preimplantation loss was noted at 3.2 times the human dose (mmol/m2 basis). Compared to untreated controls, rates of postimplantation loss and absorption increased and litter size decreased when pregnant rabbits received gadoxetate disodium at doses 26 times the recommended human single dose (mmol/m2 basis). This occurred without evidence of maternal toxicity. Because pregnant animals received repeated daily doses of gadoxetate disodium, their overall exposure was significantly higher than that achieved with the standard single dose administered to humans.
8.2 Lactation
Risk Summary
There is no information regarding the presence of gadoxetate disodium in human milk, the effects of the drug in a breastfed infant, or the effects of the drug on milk production. However, published lactation data on other GBCAs report that 0.01 to 0.04% of the maternal gadolinium dose is present in breast milk and there is limited GBCA gastrointestinal absorption in the breastfed infant. In rat lactation studies with [153Gd] gadoxetate disodium, less than 0.5% of the total administered radioactivity was transferred to the nursing pup.
Clinical Considerations
A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for up to 10 hours after EOVIST administration in order to minimize exposure to a breastfed infant.
Data
Animal Data
In lactating rats given 0.1 mmol/kg [153Gd] gadoxetate disodium, less than 0.5% of the total administered radioactivity was transferred to the neonates via maternal milk, mostly within 2 hours.
8.4 Pediatric Use
Adequate and well-controlled studies of EOVIST in pediatric patients have not been conducted. An observational study with EOVIST was performed in 52 patients (aged > 2 months and < 18 years) referred for evaluation of suspected or known focal liver lesions. EOVIST improved border delineation and increased contrast of the primary lesion in the majority of patients when compared to non-contrast images. No safety issues were identified.
No dose adjustment according to age is necessary in pediatric patients. The safety and effectiveness of EOVIST have not been established in premature infants.
NSF Risk
No case of NSF associated with EOVIST or any other GBCA has been identified in pediatric patients ages 6 years and younger.
Juvenile Animal Data
Single and repeat-dose toxicity studies in neonatal and juvenile rats did not reveal findings suggestive of a specific risk for use in pediatric patients including term neonates and infants.
8.5 Geriatric Use
In clinical studies of EOVIST, 674 (34%) patients were 65 years of age and over, while 20 (1%) were 80 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, use of EOVIST in an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
In a clinical pharmacology study, slight to moderate differences in pharmacokinetic parameters of gadoxetate disodium (increased AUC and terminal half-life, decreased total clearance) were found in a group of geriatric volunteers in comparison to non-geriatric volunteers. No clinically relevant differences in liver contrast enhancement were found.
8.6 Renal Impairment
In a clinical pharmacology study in a group of patients with moderate renal impairment, a moderate increase in AUC and terminal half-life was observed in comparison to healthy volunteers with normal renal function. Hepatic contrast did not differ among the groups.
End-stage renal failure may impair EOVIST imaging performance [see Warnings and Precautions (5.6)]. In a study of patients with end-stage renal failure, the terminal half-life was prolonged about 12-fold and the AUC was increased about 6-fold. Hepatic contrast was markedly reduced in these patients, which was attributed to significantly elevated serum ferritin levels [see Warnings and Precautions (5.1)].Approximately 30% of the injected dose was removed by dialysis in a single 3-hour dialysis session, which started one hour after an EOVIST dose. EOVIST was almost completely eliminated via dialysis and biliary excretion within the observation period of 6 days, predominantly within the first 3 days.
8.7 Hepatic Impairment
In a clinical pharmacology study in groups of patients with mild or moderate hepatic impairment, a slight to moderate increase in plasma AUC, half-life and urinary excretion, as well as decrease in hepatobiliary excretion was observed in comparison to healthy subjects with normal liver function. Hepatic contrast signal did not differ among the groups.
Severe hepatic impairment may impair EOVIST imaging performance [see Warnings and Precautions (5.6)].In patients with severe hepatic impairment, especially in patients with abnormally high (> 3 mg/dL) serum bilirubin levels, the AUC was increased up to 60% and the elimination half-life was increased up to 49%. The hepatobiliary excretion substantially decreased to about 5% of the administered dose and reduced hepatic contrast signal was observed.
A dose adjustment is not necessary for patients with hepatic impairment.
In clinical studies, 489 patients had a diagnosis of liver cirrhosis (Child-Pugh category A, n = 270; category B, n = 98; category C, n = 24; unknown category, n = 97). No difference in diagnostic performance and safety was observed among these patients.
-
10 OVERDOSAGE
The maximum dose studied in MR imaging was 0.4 mL/kg (0.1 mmol/kg) body weight and was tolerated in a manner similar to lower doses. In case of inadvertent overdosage in patients with severely impaired renal and/or hepatic function, EOVIST can be partially removed by hemodialysis [see Use in Specific Populations (8.6)].
-
11 DESCRIPTION
EOVIST (gadoxetate disodium) is a paramagnetic contrast agent administered for MRI. EOVIST is provided as a sterile, clear, colorless to pale yellow aqueous solution for intravenous injection.
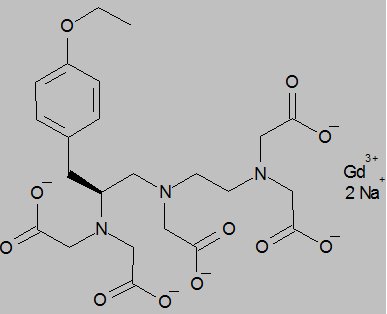
EOVIST contains the active pharmaceutical ingredient, gadoxetate disodium (Gd‑EOB‑DTPA). The chemical name for gadoxetate disodium is (4S)-4-(4-Ethoxybenzyl)-3,6,9-tris(carboxylatomethyl)-3,6,9-triazaundecanedioic acid, gadolinium complex, disodium salt. Gadoxetate disodium has a molecular weight of 725.72 and an empirical formula of GdC23H28N3O11Na2. The structural formula of gadoxetate disodium in aqueous solution is:
Each mL of EOVIST contains 181.43 mg of gadoxetate disodium (equivalent to 0.25 mmol/mL gadoxetate disodium) and the excipients caloxetate trisodium, trometamol, hydrochloric acid and/or sodium hydroxide (for pH adjustment), and water for injection. EOVIST contains no antimicrobial preservative.
Pertinent physiochemical properties of EOVIST are provided in Table 2.
Table 2 Physicochemical Properties Osmolality at 37°C (Osm/kg H2O)
0.688
Viscosity at 37°C (cP)
1.19
- Density at 37°C (g/mL)
1.088
pH
6.8-8
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gadoxetate disodium is a paramagnetic compound and develops a magnetic moment when placed in a magnetic field. The relatively large magnetic moment produced by gadoxetate disodium results in a local magnetic field, yielding enhanced relaxation rates (shortening of relaxation times) of water protons in the vicinity of the paramagnetic agent, which leads to an increase in signal intensity (brightening) of blood and tissue.
In MRI, visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occur with 1) differences in proton density; 2) differences of the spin-lattice or longitudinal relaxation times (T1); and 3) differences in the spin-spin or transverse relaxation time (T2). When placed in a magnetic field, gadoxetate disodium decreases the T1 and T2 relaxation time in target tissue. At the recommended dose, the effect is observed with greatest sensitivity in T1-weighted MR sequences.
12.2 Pharmacodynamics
EOB-DTPA forms a stable complex with the paramagnetic gadolinium ion with a thermodynamic stability of log KGdL=‑23.46. Gadoxetate disodium is a highly water-soluble, hydrophilic compound with a lipophilic moiety, the ethoxybenzyl group (EOB). Gadoxetate disodium shows a weak (<10%), transient protein binding and the relaxivity in plasma is about 8.7 L/mmol/sec at pH 7, 39°C and 0.47 T.
Gadoxetate disodium is selectively taken up by hepatocytes [see Clinical Pharmacology (12.3)] resulting in increased signal intensity in liver tissue [see Dosage and Administration (2.3)].
EOVIST exhibits a biphasic mode of action: first, distribution in the extracellular space after bolus injection and subsequently, selective uptake by hepatocytes (and biliary excretion) due to the lipophilic (EOB) moiety.
12.3 Pharmacokinetics
Distribution
After intravenous administration, the plasma concentration time profile of gadoxetate disodium is characterized by a bi-exponential decline. The total distribution volume of gadoxetate disodium at steady state is about 0.21 L/kg (extracellular space); plasma protein binding is less than 10%. Following GBCA administration, gadolinium is present for months or years in brain, bone, skin, and other organs [see Warnings and Precautions (5.3)].
Elimination
Gadoxetate disodium is equally eliminated via the renal and hepatobiliary routes. The mean terminal elimination half-life of gadoxetate disodium (0.01 to 0.1 mmol/kg) has been observed in healthy volunteers of 22–39 years of age to be 0.91 to 0.95 hour. Clearance appeared to decrease slightly with increasing age. The pharmacokinetics are dose-linear up to a dose of 0.4 mL/kg (0.1 mmol/kg), which is 4 times the recommended dose [see Use in Specific Populations (8.4, 8.5, 8.6, and8.7)].
A total serum clearance (Cltot) was 250 mL/min, whereas the renal clearance (Clr) corresponds to about 120 mL/min, a value similar to the glomerular filtration rate in healthy subjects.
Metabolism
Gadoxetate disodium is not metabolized.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies of EOVIST have been conducted.
Gadoxetate disodium was not mutagenic in in vitro reverse mutation tests in bacteria, or in chromosome aberration tests in human peripheral blood lymphocytes, and was negative in an in vivo micronucleus test in mice after intravenous injection of doses up to 4 mmol/kg.
Gadoxetate disodium had no effect on fertility and general reproductive performance of male and female rats when given in doses 6.5 times the human dose (based on body surface area).
13.2 Animal Toxicology and/or Pharmacology
A dose-related increase in QTc which was resolved by 30 minutes post dosing was observed in dogs when given a single dose of EOVIST. The increase was noted when given in doses equal to or greater than 0.1 mmol/kg (2.2 times the human dose). Maximum increase in QTcF was equal to or less than 20 ms at doses up to 0.5 mmol/kg (11 times the human dose).
A gait disturbance was observed in 1 of 3 mice when given EOVIST at a dose of approximately 1.1 mmol/kg (3.6 times the human dose); the disturbance occurred at 30 minutes post dosing and resolved at 4 hours post dosing.
Local intolerance reactions, including moderate interstitial hemorrhage, edema, and focal muscle fiber necrosis, were observed after intramuscular administration of EOVIST [see Warning and Precautions (5.5)].
-
14 CLINICAL STUDIES
Patients with suspected or known focal liver lesions were enrolled in two of four non-randomized, intrapatient-controlled studies that evaluated predominantly the detection (studies 1 and 2) or morphological characterization (studies 3 and 4) of liver lesions. Studies 1 and 2 ("detection" studies) enrolled patients who were scheduled for liver surgery. MRI results were compared to a reference standard that consisted of surgical histopathology and the results from intra-operative ultrasound of the liver. The studies assessed the sensitivity of pre-contrast MRI and EOVIST-contrasted MRI for the detection of liver lesions, when each set of images was compared to the reference.
Studies 3 and 4 ("characterization" studies) enrolled patients with known or suspected focal liver lesions, including patients who were not scheduled for liver surgery. MRI results were compared to a reference standard that consisted of surgical histopathology and other prospectively defined criteria. The studies assessed the correctness of liver lesion characterization by pre-contrast MRI and EOVIST-contrasted MRI, when each set of images was compared to the reference. Lesions were characterized as one of the following choices: hepatocellular carcinoma, cholangiocarcinoma, metastasis, focal lymphoma, adenoma, focal nodular hyperplasia, hemangioma, abscess, focal liver fibrosis, regenerative nodule, focal fat, hydatid cyst, liver cyst, "not assessable", normal, no lesion or "other."
In all four studies, patients underwent a baseline, pre-contrast MRI followed by the administration of EOVIST at a dose of 0.025 mmol/kg, with MRI performed immediately (the "dynamic" phase) and at 10 to 20 minutes following EOVIST administration (the "hepatocyte" phase). Patients also underwent computerized tomography with contrast examinations of the liver. Pre-contrast MRI and EOVIST-contrasted MR images were evaluated in a systematic, randomized, paired and unpaired fashion by three radiologists who were blinded to clinical information. CT images were also evaluated by the radiologists in a separate reading session.
Diagnostic efficacy was determined in 621 patients. The average age was 57 years (range 19 to 84 years) and 54% were male. The ethnic representations were 90% Caucasian, 4% Black, 3% Hispanic, 2% Asian, and 1% of other ethnic groups.
The combination of non-contrasted and EOVIST-contrasted MR images had improved sensitivity for the detection and characterization of liver lesions, compared to pre-contrasted MR images (Tables 3 and 4). The improved sensitivity in detection of lesions was predominantly related to the detection of additional lesions among patients with multiple lesions on the pre-contrast MR images. The false positive rates for detection of lesions were similar for non-contrasted MR images and EOVIST-contrasted MR images (32% versus 34%, respectively). Liver lesion detection and characterization results were similar between CT and the combination of pre-contrasted and EOVIST-contrasted MR images.
Table 3 Sensitivity in Liver Lesion Detection Diagnostic Procedure
Reader
Study 1
Sensitivity (%)
n=129Study 2
Sensitivity (%)
n=126Pre-contrast MRI
Reader 1
76
77
Reader 2
76
73
Reader 3
71
72
Combined pre- and EOVIST-contrast MRI
Reader 1
81
82
Reader 2
78
76
Reader 3
74
78
Difference:
combined pre + EOVIST-contrast MRI minus pre MRI
(95% confidence interval)
Reader 1
5 (1, 9)*
5 (1, 9)*
Reader 2
2 (-1, 5)
3 (-1, 7)
Reader 3
3 (0, 6)*
6 (0, 10)*
* Statistically significant improvement
Table 4 Proportion of Correctly Characterized Lesions Study 3
Study 4
Diagnostic Procedure
Reader
n
Proportion correct (%) **
n
Proportion correct (%) **
Pre-contrast MRI
Reader 1
182
51
177
60
Reader 2
182
59
177
64
Reader 3
182
53
177
48
Combined pre- and EOVIST-contrast MRI
Reader 1
182
67
177
61
Reader 2
182
76
177
76
Reader 3
182
58
177
67
Difference: combined pre- and EOVIST-contrast MRI minus
pre-contrast MRI(95% confidence interval)
Reader 1
16 (7, 25)*
1 (-7, 10)
Reader 2
17 (9, 25)*
11 (5, 18)*
Reader 3
5 (-2, 12)
19 (11, 27)*
* Statistically significant improvement
** Proportion of correctly characterized lesions with respect to the reference
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
EOVIST is supplied in single-dose, rubber stoppered containers (vials) containing 181.43 mg/mL of gadoxetate disodium (equivalent to 0.25 mmol/mL gadoxetate disodium), in the following sizes:
- 10 mL single-dose containers (vials) filled with 10 mL, boxes of 5 (NDC: 50419-320-05)
- 15 mL single-dose containers (vials) filled with 15 mL, boxes of 5 (NDC: 50419-320-15)
16.2 Storage and Handling
Store at temperatures between 20 to 25° C (68 to 77° F); excursions permitted to 15 to 30° C [see USP Controlled Room Temperature].
EOVIST is a ready-to-use solution for single use only. Visually inspect EOVIST for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored or if particulate matter is present. The rubber stopper should not be pierced more than once. Use EOVIST immediately after opening. Unused portions should be discarded.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Nephrogenic Systemic Fibrosis
Instruct patients to inform their physician if they:
- Have a history of kidney disease and/or liver disease
- Have recently received a GBCA
GBCAs increase the risk of NSF among patients with impaired elimination of drugs. To counsel patients at risk of NSF:
- Describe the clinical manifestations of NSF
- Describe procedures to screen for the detection of renal impairment
Instruct the patients to contact their physician if they develop signs or symptoms of NSF following EOVIST administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness.
Common Adverse Reactions
Inform patients that they may experience:
- Reactions along the venous injection site, such as mild and transient burning or pain or feeling of warmth or coldness at the injection site
- Side effects of headache, nausea, abnormal taste and feeling hot
General Precautions
Gadolinium Retention
- Advise patients that gadolinium is retained for months or years in brain, bone, skin, and other organs in patients with normal renal function. The clinical consequences of retention are unknown. Retention depends on multiple factors and is greater following administration of linear GBCAs than following administration of macrocyclic GBCAs [see Warnings and Precautions (5.3)].
Instruct patients receiving EOVIST to inform their physician if they:
- Are pregnant or breastfeeding
- Have a history of allergic reaction to contrast media, bronchial asthma or allergic respiratory disorder
© 2008, Bayer HealthCare Pharmaceuticals Inc., All rights reserved.
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981Manufactured in Germany
-
MEDICATION GUIDE
Medication Guide
EOVIST
(e-o-vist)(gadoxetate disodium)
Injection for intravenous useWhat is Eovist?
- Eovist is a prescription medicine called a gadolinium-based contrast agent (GBCA). Eovist, like other GBCAs, is injected into your vein and used with a magnetic resonance imaging (MRI) scanner.
- An MRI exam with a GBCA, including Eovist, helps your doctor to see problems better than an MRI exam without a GBCA. Eovist is needed to better see the problems in your liver.
- Your doctor has reviewed your medical records and has determined that you would benefit from using a GBCA with your MRI exam.
What is the most important information I should know about Eovist?
- Eovist contains a metal called gadolinium. Small amounts of gadolinium can stay in your body including the brain, bones, skin and other parts of your body for a long time (several months to years).
- It is not known how gadolinium may affect you, but so far, studies have not found harmful effects in patients with normal kidneys.
- Rarely, patients have reported pains, tiredness, and skin, muscle or bone ailments for a long time, but these symptoms have not been directly linked to gadolinium.
- At equivalent doses, the amount of gadolinium that stays in the body is different for different gadolinium medicines. Gadolinium stays in the body more after Omniscan or Optimark than after Eovist, Magnevist, or MultiHance. Gadolinium stays in the body the least after Dotarem, Gadavist, or ProHance.
- People who get many doses of gadolinium medicines, women who are pregnant and young children may be at increased risk from gadolinium staying in the body.
- Some people with kidney problems who get gadolinium medicines can develop a condition with severe thickening of the skin, muscles and other organs in the body (nephrogenic systemic fibrosis). Your healthcare provider should screen you to see how well your kidneys are working before you receive Eovist.
Do not receive Eovist if you have had a severe allergic reaction to Eovist.
Before receiving Eovist, tell your healthcare provider about all your medical conditions, including if you:
- have had any MRI procedures in the past where you received a GBCA. Your healthcare provider may ask you for more information including the dates of these MRI procedures.
- are pregnant or plan to become pregnant. It is not known if Eovist can harm your unborn baby. Talk to your healthcare provider about the possible risks to an unborn baby if a GBCA such as Eovist is received during pregnancy.
- have kidney problems, diabetes, or high blood pressure.
- have had an allergic reaction to dyes (contrast agents) including GBCAs
What are the possible side effects of Eovist?
- See “What is the most important information I should know about Eovist?”
- Allergic reactions. Eovist can cause allergic reactions that can sometimes be serious. Your healthcare provider will monitor you closely for symptoms of an allergic reaction.
The most common side effects of Eovist include: nausea, headache, feeling hot, dizziness, and back pain.
These are not all the possible side effects of Eovist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of EOVIST.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider for information about EOVIST that is written for health professionals.
What are the ingredients in Eovist?
Active ingredient: gadoxetate disodium
Inactive ingredients: caloxetate trisodium, trometamol, hydrochloric acid and/or sodium hydroxide (for pH adjustment), and water for injection.
Manufactured for Bayer HealthCare Pharmaceuticals Inc.
Manufactured in Germany
© 2008 Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
For more information, go to www.eovist.com or call 1-888-842-2937.
-
Carton 5 x 10 mL
NDC: 50419-320-05
5 vials of 10 mL
sterile solution
Eovist® 10 mL
(gadoxetate disodium)
Injection
0.25 mol/L
Rx only
Each mL contains 181.43 gadoxetate disodium and the excipients caloxetate trisodium, trometamol, hydrochloric acid and/or sodium hydroxide (for pH adjustment), and water for injection. Eovist® contains no antimicrobial preservative.
Single-dose container.
Discard unused portion.
Store at 20-25°C (68-77°F); excursions permitted to 15-30°C [See USP Controlled Room Temperature.]
For intravenous administration.
Dosage: See package insert.
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd. in Germany
-
INGREDIENTS AND APPEARANCE
EOVIST
gadoxetate disodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-320 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GADOXETATE DISODIUM (UNII: HOY74VZE0M) (GADOLINIUM CATION (3+) - UNII:AZV954TZ9N) GADOXETATE DISODIUM 181.43 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-320-05 5 in 1 BOX 07/03/2008 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 50419-320-15 5 in 1 BOX 07/03/2008 2 15 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 3 NDC: 50419-320-75 5 in 1 BOX 07/03/2008 3 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022090 07/03/2008 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Bayer Pharma AG 315015982 MANUFACTURE(50419-320) Establishment Name Address ID/FEI Business Operations QUALITY PACKAGING SPECIALISTS INTERNATIONAL LLC 080629831 RELABEL(50419-320)
Trademark Results [EOVIST]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EOVIST 76358237 2758129 Live/Registered |
BAYER INTELLECTUAL PROPERTY GMBH 2002-01-14 |
 EOVIST 74668016 not registered Dead/Abandoned |
Schering Aktiengesellschaft 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.