CVS Health Lip Moisture by CVS Pharmacy Drug Facts
CVS Health Lip Moisture by
Drug Labeling and Warnings
CVS Health Lip Moisture by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CVS HEALTH LIP MOISTURE MINT - octinoxate, oxybenzone, white petrolatum stick
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
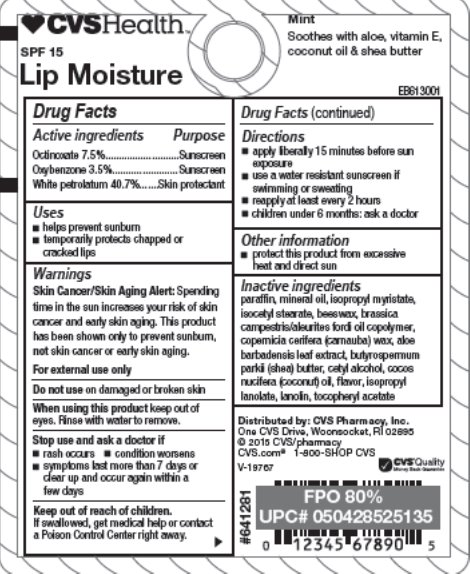
Drug Facts
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
Other information
- protect this product from excessive heat and direct sun
Inactive ingredients
paraffin, mineral oil, isopropyl myristate, isocetyl stearate, beeswax, brassica campestris/aleurites fordi oil copolymer, copernicia cerifera (carnauba) wax, aloe barbadensis leaf extract, buyrospermum parkii (shea) butter, cetyl alcohol, cocos nucifera (coconut) oil, flavor, isopropyl lanolate, lanolin, tocopheryl acetate
| CVS HEALTH LIP MOISTURE
MINT
octinoxate, oxybenzone, white petrolatum stick |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.