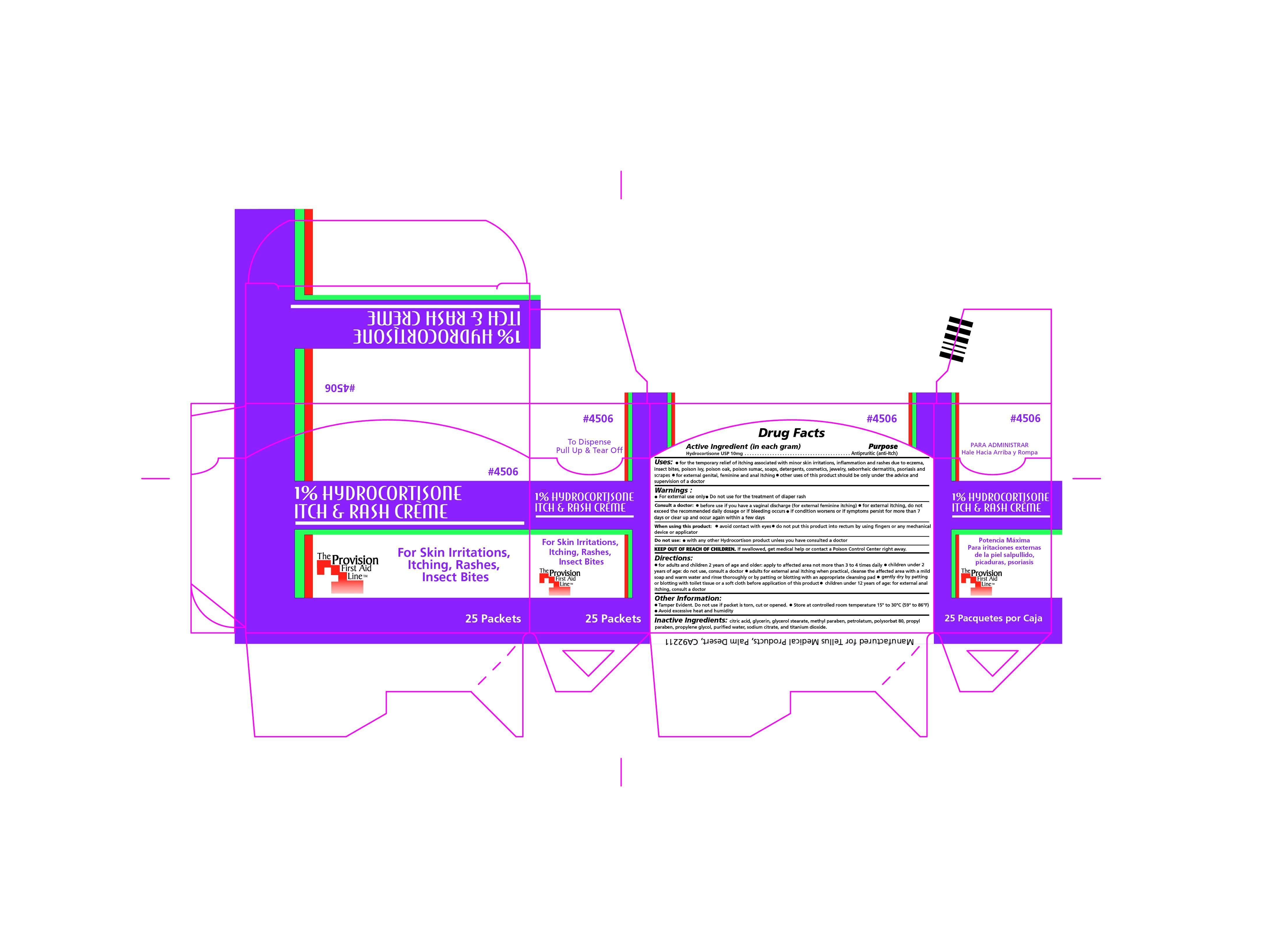
ITCH AND RASH CREAM- hydrocortisone cream
Itch and Rash Cream by
Drug Labeling and Warnings
Itch and Rash Cream by is a Otc medication manufactured, distributed, or labeled by Provision Medical Products, ULTRAtab Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
-
WARNINGS
Warnings :
For external use only Do not use for the treatment of diaper rash
Consult a doctor:before use if you have a vaginal discharge (for external feminine itching):
for external itching, do not exceed the recommended daily dosage or if bleeding occurs
if condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days
When using this product:avoid contact with eyes, do not put this product into rectum by using fingers or any mechanical
Do not use:with any other Hydrocortison product unless you have consulted a doctor -
DOSAGE & ADMINISTRATION
dIRECTIONS: fOR ADULTS AND CHILDREN 2 YEARS OF AGE AND OLDER-APPLY TO AFFECTED AREA NOT MORE THAN 3 OR 4 TIMES DAILY
CHILDREN UNDER 2 YEARS, DO NOT USE, CONSULT A DOCTOR. ADULTS FOR EXTERNAL ANAL ITCHING WHEN PRACTICAL-CLEANSE THE AFFECTED AREA WITH A MILD SOAP AND WARM WATER AND RINCE THOROUGHLY OR BY PATTING AND BLOTTING WITH AN APPROPRIATE CLEANSING PAD. GENTLY DRY BY PATTING OR BLOTTING WITH A SOFT CLOTH BEFORE APPLICATION OF THIS PRODUCT. CHILDREN UNDER 12 YEARS OF AGE-FOR EXTERNAL ANAL ITCHING, CONSULT A DOCTOR
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ITCH AND RASH CREAM
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69103-4506 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) PETROLATUM (UNII: 4T6H12BN9U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (SNOW WHITE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69103-4506-3 25 in 1 CARTON 04/03/2015 1 .9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/31/2015 Labeler - Provision Medical Products (036936831) Registrant - Provision Medical Products (036936831) Establishment Name Address ID/FEI Business Operations Ultraseal Corporation 085752004 pack(69103-4506) Establishment Name Address ID/FEI Business Operations ULTRAtab Laboratories, Inc. 151051757 manufacture(69103-4506)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.