NOURESS- cysteine hydrochloride injection
Nouress by
Drug Labeling and Warnings
Nouress by is a Prescription medication manufactured, distributed, or labeled by Avadel Legacy Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NOURESS™ safely and effectively. See full prescribing information for NOURESS.
NOURESS (cysteine hydrochloride injection), for intravenous use
Initial U.S. Approval: 1971
INDICATIONS AND USAGE
NOURESS is a sulfur-containing amino acid indicated for use as an additive to amino acids solutions to meet nutritional requirements of neonates (preterm and term infants less than one month of age) requiring total parenteral nutrition. (1)
DOSAGE AND ADMINISTRATION
- NOURESS is for intravenous infusion after dilution and admixing only. (2.1)
- See full prescribing information for information on preparation, administration, and instructions for use. (2.1, 2.2, 2.3, 2.4)
- The recommended dosage in neonates is based upon the recommended daily protein (amino acid) requirements: 22 mg NOURESS/g amino acids. The corresponding volume is 0.44 mL NOURESS/g amino acids. (2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 500 mg/10 mL (50 mg/mL) cysteine hydrochloride, USP in a single-dose vial. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Pulmonary Embolism due to Pulmonary Vascular Precipitates: If signs of pulmonary distress occur, stop the infusion and initiate a medical evaluation. (5.1)
- Vein Damage and Thrombosis: Solutions with osmolarity of 900 mOsm/L or more must be infused through a central catheter (2.1, 5.2)
- Increased Blood Urea Nitrogen (BUN): Monitor laboratory parameters and discontinue if BUN exceeds normal postprandial limits and continues to increase. (5.3)
- Acid-Base Imbalance: Monitor laboratory parameters and supplement with electrolytes as needed. (5.4)
- Hepatobiliary Disorders: Neurocognitive delay possible in infants; monitor liver function parameters and ammonia levels. (5.5, 8.4)
- Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm infants. (5.6, 8.4)
- Monitoring and Laboratory Tests: Monitor fluid and electrolytes, serum osmolarity, blood glucose, kidney and liver function, blood count, and coagulation parameters throughout treatment. (5.7)
ADVERSE REACTIONS
Most common adverse reactions are local reactions (warm sensation, erythema, phlebitis, and thrombosis at the infusion site), generalized flushing, fever, nausea, and metabolic acidosis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Avadel at 1-877-638-4579 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Preparation and Administration Information
2.3 Preparation Instructions for Admixing Using a Parenteral Nutrition Container
2.4 Dosing Considerations
2.5 Recommended Dosage for Neonates
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pulmonary Embolism due to Pulmonary Vascular Precipitates
5.2 Vein Damage and Thrombosis
5.3 Increased Blood Urea Nitrogen (BUN)
5.4 Acid-Base Imbalance
5.5 Hepatobiliary Disorders
5.6 Aluminum Toxicity
5.7 Monitoring and Laboratory Tests
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
NOURESS is for intravenous infusion after dilution and admixing use only. Prior to administration, NOURESS must be diluted and used as an admixture in parenteral nutrition solutions.
The resulting solution is for intravenous infusion into a central or peripheral vein. The choice of a central or peripheral venous route should depend on the osmolarity of the final infusate. Solutions with osmolarity of 900 mOsm/L or greater must be infused through a central catheter [see Warnings and Precautions (5.2)].
2.2 Preparation and Administration Information
- Prior to administration, NOURESS must be diluted and used as an admixture in parenteral nutrition solutions.
- NOURESS is to be prepared only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area). The key factor in the preparation is careful aseptic technique to avoid inadvertent touch contamination during mixing of solutions and addition of other nutrients.
- NOURESS is for addition to amino acid solutions prior to further admixing with dextrose injection using a parenteral nutrition container.
- Calcium and phosphate ratios must be considered. Excess addition of calcium and phosphate, especially in the form of mineral salts, may result in the formation of calcium phosphate precipitates [see Warnings and Precautions (5.1)].
- Use a dedicated line for parenteral nutrition solutions.
- Intravenous lipid emulsions can be infused concurrently into the same vein as NOURESS-containing amino acid and dextrose solutions by a Y-connector located near the infusion site; flow rates of each solution should be controlled separately by infusion pumps.
- For administration, use a 0.22 micron in-line filter.
- To prevent air embolism, use a non-vented infusion set or close the vent on a vented set, avoid multiple connections, do not connect flexible containers in series, fully evacuate residual gas in the container prior to administration, do not pressurize the flexible container to increase flow rates, and if administration is controlled by a pumping device, turn off pump before the container runs dry.
- Visually inspect the diluted parenteral nutrition solution containing NOURESS for particulate matter and discoloration before admixing, after admixing, after removal from refrigeration, and prior to administration. The solution should be clear and there should be no precipitates. A slight yellow color does not alter the quality and efficacy of this product.
2.3 Preparation Instructions for Admixing Using a Parenteral Nutrition Container
- Remove NOURESS vial from the carton and inspect for particulate matter.
- Transfer the required amount of NOURESS to an amino acid solution using strict aseptic techniques to avoid microbial contamination.
- The amino acid solution containing NOURESS can then be used to prepare admixtures in the parenteral nutrition container using strict aseptic techniques.
- Amino acids solution containing NOURESS may be mixed with dextrose injection. The following proper mixing sequence must be followed to minimize pH related problems:
- Transfer dextrose injection to the parental nutrition pooling container
- Transfer phosphate salt
- Transfer NOURESS-containing amino acid solution
- Transfer electrolytes
- Transfer trace elements
- Use gentle agitation during admixing to minimize localized concentration effects; shake containers gently after each addition.
- For automated compounding, refer to Instructions for Use of the applicable compounder.
- Because additives may be incompatible, evaluate all additions to the parenteral nutrition container for compatibility and stability of the resulting preparation. Consult with a pharmacist, if available. Questions about compatibility may be directed to Avadel. If it is deemed advisable to introduce additives to the parenteral nutrition container, use aseptic technique.
- Inspect the final parenteral nutrition solution containing NOURESS to ensure that precipitates have not formed during mixing or on addition of additives. Discard if any precipitates are observed.
Stability and Storage
- For single use only. Discard unused portion of the vial of NOURESS.
- Use parenteral nutrition solution containing NOURESS promptly after mixing. Any storage of the admixture should be under refrigeration at 2°C to 8°C (36°F to 46°F) and limited to a brief period of time, no longer than 24 hours. After removal from refrigeration, inspect for precipitates, use promptly, and complete the infusion within 24 hours. Discard if any precipitates are observed.
- Discard any remaining admixture.
- Protect parenteral nutrition solution from light.
2.4 Dosing Considerations
The dosage of the final parenteral nutrition solution containing NOURESS must be based on the concentrations of all components in the solution and the recommended nutritional requirements [see Dosage and Administration (2.5)]. Consult the prescribing information of all added components to determine the recommended nutritional requirements.
The dosage of NOURESS should be individualized based on the patient’s clinical condition (ability to adequately metabolize amino acids), body weight and nutritional/fluid requirements, as well as additional energy given orally/enterally to the patient. Prior to initiating parenteral nutrition, the following patient information should be reviewed: review of all medications, gastrointestinal function and laboratory data (such as electrolytes (including magnesium, calcium, and phosphorus), glucose, urea/creatinine, liver panel, and complete blood count).
Prior to administration of parenteral nutrition solution containing NOURESS, correct severe fluid, electrolyte and acid-base disorders.
2.5 Recommended Dosage for Neonates
The recommended dosage and volume of NOURESS is based upon the recommended daily protein (amino acid) requirements.
Table 1. Recommended Daily Dosage of NOURESS in Neonates (Preterm and Term Infants Less Than One Month of Age) a Protein is provided as amino acids.
Dosage Proteina Requirement
(g Amino Acids/kg/day)1Dosage
(mg NOURESS/g Amino Acids)Volume
(mL NOURESS/g Amino Acids)Neonates 3 to 4 22 0.44 NOURESS contains 50 mg/mL of cysteine hydrochloride (equivalent to 34.5 mg/mL of cysteine). Therefore, the recommended dosage of NOURESS provides 15 mg cysteine/gram of amino acids for neonates.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Pulmonary Embolism due to Pulmonary Vascular Precipitates
Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving parenteral nutrition. In some fatal cases, pulmonary embolism occurred as a result of calcium phosphate precipitates. Precipitation following passage through an in-line filter and suspected in vivo precipitate formation has also been reported. If signs of pulmonary distress occur, stop the parenteral nutrition infusion and initiate a medical evaluation. In addition to inspection of the solution [see Dosage and Administration (2.1, 2.2)], the infusion set and catheter should also periodically be checked for precipitates.
5.2 Vein Damage and Thrombosis
NOURESS must be diluted and used as an admixture in parenteral nutrition solutions. Solutions with an osmolarity of 900 mOsm/L or greater must be infused through a central catheter [see Dosage and Administration (2.1)]. The infusion of hypertonic nutrient injections into a peripheral vein may result in vein irritation, vein damage, and/or thrombosis. The primary complication of peripheral access is venous thrombophlebitis, which manifests as pain, erythema, tenderness or a palpable cord. Remove the catheter as soon as possible, if thrombophlebitis develops.
5.3 Increased Blood Urea Nitrogen (BUN)
Intravenous infusion of amino acids may induce a rise in blood urea nitrogen (BUN), especially in patients with impaired hepatic or renal function. Appropriate laboratory tests should be performed periodically and infusion discontinued if BUN levels exceed normal postprandial limits and continue to rise. It should be noted that a modest rise in BUN normally occurs as a result of increased protein intake.
Administration of amino acid solutions in the presence of impaired renal function may augment an increasing BUN, as does any protein dietary component.
5.4 Acid-Base Imbalance
Administration of NOURESS may result in metabolic acidosis in neonates.
Administration of amino acid solutions to a patient with hepatic impairment may result in serum amino acid imbalances, metabolic alkalosis, prerenal azotemia, hyperammonemia, stupor and coma.
Frequent clinical evaluation and laboratory determinations are necessary for proper monitoring of acid-base balance during parenteral nutrition therapy. Significant deviations from normal concentrations may require the use of additional electrolyte supplements.
5.5 Hepatobiliary Disorders
Hepatobiliary disorders are known to develop in some patients, including neonates, without preexisting liver disease who receive parenteral nutrition, including cholecystitis, cholelithiasis, cholestasis, hepatic steatosis, fibrosis and cirrhosis, possibly leading to hepatic failure. The etiology of these disorders is thought to be multifactorial and may differ between patients.
Instances of asymptomatic hyperammonemia have been reported in patients receiving parenteral nutrition without overt liver dysfunction. The mechanisms of this reaction are not clearly defined but may involve genetic defects and immature or subclinically impaired liver function [see Contraindications (4), Use in Specific Populations (8.4)].
Hyperammonemia is of special significance in infants, as it can result in neurocognitive delays. Monitor liver function parameters and ammonia levels during treatment with NOURESS. Patients developing signs of hepatobiliary disorders should be assessed early by a clinician knowledgeable in liver diseases in order to identify possible causative and contributory factors, and possible therapeutic and prophylactic interventions.
5.6 Aluminum Toxicity
NOURESS contains aluminum that may be toxic.
Aluminum may reach toxic levels with prolonged parenteral administration in patients with renal impairment. Neonates and preterm infants are particularly at risk for aluminum toxicity because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which also contain aluminum.
Patients with renal impairment, including neonates and preterm infants, who receive greater than 4 to 5 mcg/kg/day of parenteral aluminum can accumulate aluminum to levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Exposure to aluminum from NOURESS is not more than 0.25 mcg/kg/day when preterm and term neonates are administered the recommended maximum dosage of NOURESS (22 mg cysteine hydrochloride/g of amino acids and 4 g of amino acids/kg/day) [see Dosage and Administration (2.5)]. When prescribing NOURESS for use in parenteral nutrition containing other small volume parenteral products, the total daily patient exposure to aluminum from the admixture should be considered and maintained at no more than 5 mcg/kg/day [see Use in Specific Populations (8.4)].
5.7 Monitoring and Laboratory Tests
Monitor fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, ammonia levels, blood count and coagulation parameters throughout treatment [see Dosage and Administration (2.4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information:
- Pulmonary embolism due to pulmonary vascular precipitates [see Warnings and Precautions (5.1)]
- Vein damage and thrombosis [see Warnings and Precautions (5.2)]
- Increased BUN [see Warnings and Precautions (5.3)]
- Acid-base imbalance [see Warnings and Precautions (5.4)]
- Hepatobiliary disorders [see Warnings and Precautions (5.5)]
- Aluminum toxicity [see Warnings and Precautions (5.6)]
Adverse reactions with the use of cysteine hydrochloride injection were identified in clinical studies or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
- Metabolic acidosis
- Local infusion site reactions, including a warm sensation, erythema, phlebitis, and thrombosis at the infusion site
- Generalized flushing, fever, and nausea
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
NOURESS for use as an additive to amino acid solutions to meet nutritional requirements is not indicated for use in adults. Appropriate administration of NOURESS is not expected to cause major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with cysteine hydrochloride.
8.2 Lactation
Risk Summary
NOURESS is used as an additive to amino acid solutions to meet nutritional requirements of neonates requiring total parenteral nutrition and is not indicated for use in adults. There are no data on the presence of cysteine hydrochloride in human or animal milk or the effects on milk production. Data available on the effects of cysteine hydrochloride on infants, either directly or through breastmilk, do not suggest a significant risk of adverse reactions from exposure.
8.4 Pediatric Use
NOURESS is indicated for use as an additive to amino acid solutions to meet the nutritional requirements of neonates, including preterm infants, requiring total parenteral nutrition. The safety profile for NOURESS use in neonates includes risks of acid-base imbalance and hepatobiliary dysfunction.
Acid-base imbalance, including metabolic acidosis, and liver dysfunction may occur with NOURESS administration in preterm infants. Frequent clinical and laboratory assessments are necessary to monitor and manage fluid balance, electrolyte concentrations, liver tests, and acid-base balance during parenteral nutrition therapy [see Warnings and Precautions (5.4)].
Hyperammonemia is of special significance in neonates. This reaction appears to be related to a deficiency of the urea cycle amino acids of genetic or product origin. It is essential that blood ammonia be measured during treatment [see Warnings and Precautions (5.5)].
Because of immature renal function, neonates including preterm infants, receiving prolonged parenteral nutrition with NOURESS may be at higher risk of aluminum toxicity [see Warnings and Precautions (5.7)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
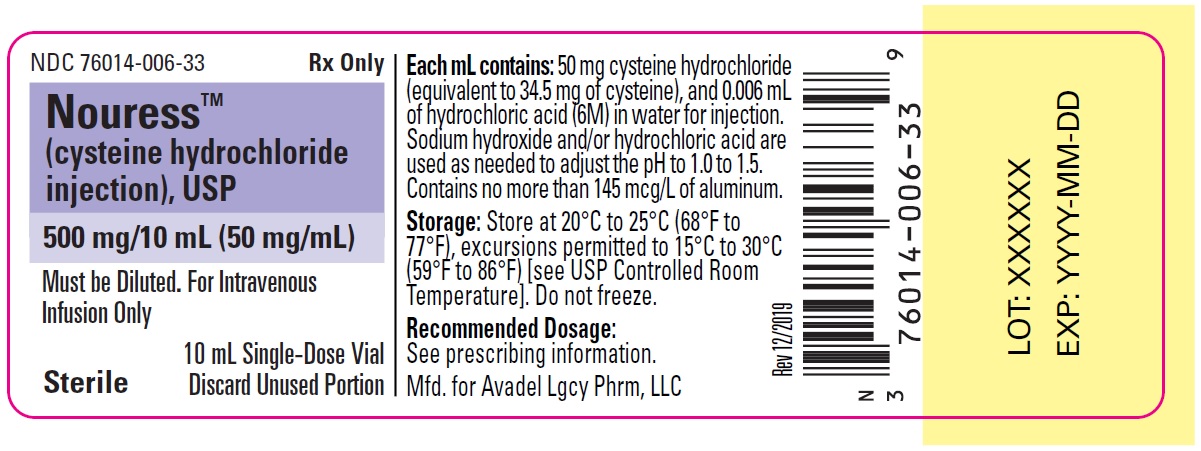
NOURESS (cysteine hydrochloride injection) is a sterile, nonpyrogenic solution for intravenous use supplied as 500 mg/10 mL cysteine hydrochloride, USP in a single-dose vial.
Each mL of NOURESS contains 50 mg of cysteine hydrochloride, (equivalent to 34.5 mg of cysteine), and 0.006 mL of hydrochloric acid (6M) in water for injection. Sodium hydroxide and/or hydrochloric acid are used as needed to adjust the pH. The pH range of NOURESS is 1.0 to 1.5.
The active ingredient is cysteine hydrochloride. The chemical name of cysteine hydrochloride is L-cysteine hydrochloride monohydrate. Its molecular formula is C3H7NO2S HCI H2O and molecular weight is 175.63. The chemical structure of L-cysteine hydrochloride monohydrate is depicted below:
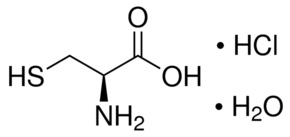
Cysteine hydrochloride is a white crystalline powder soluble in water. Cysteine is a sulfur-containing amino acid and is prone to oxidation when exposed to air in aqueous solution, which may convert cysteine to insoluble cystine resulting in precipitation over time.
NOURESS contains no more than 145 mcg/L of aluminum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous cysteine is synthesized from methionine by the enzyme, cystathionase, via the trans-sulfuration pathway, and serves as a precursor substrate for both glutathione and taurine. NOURESS provides cysteine to the systemic circulation of neonates who require parenteral nutrition and cannot synthesize adequate quantities of cysteine due to deficient cystathionase activity.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NOURESS (cysteine hydrochloride injection) is a clear, colorless, sterile and nonpyrogenic solution supplied as follows:
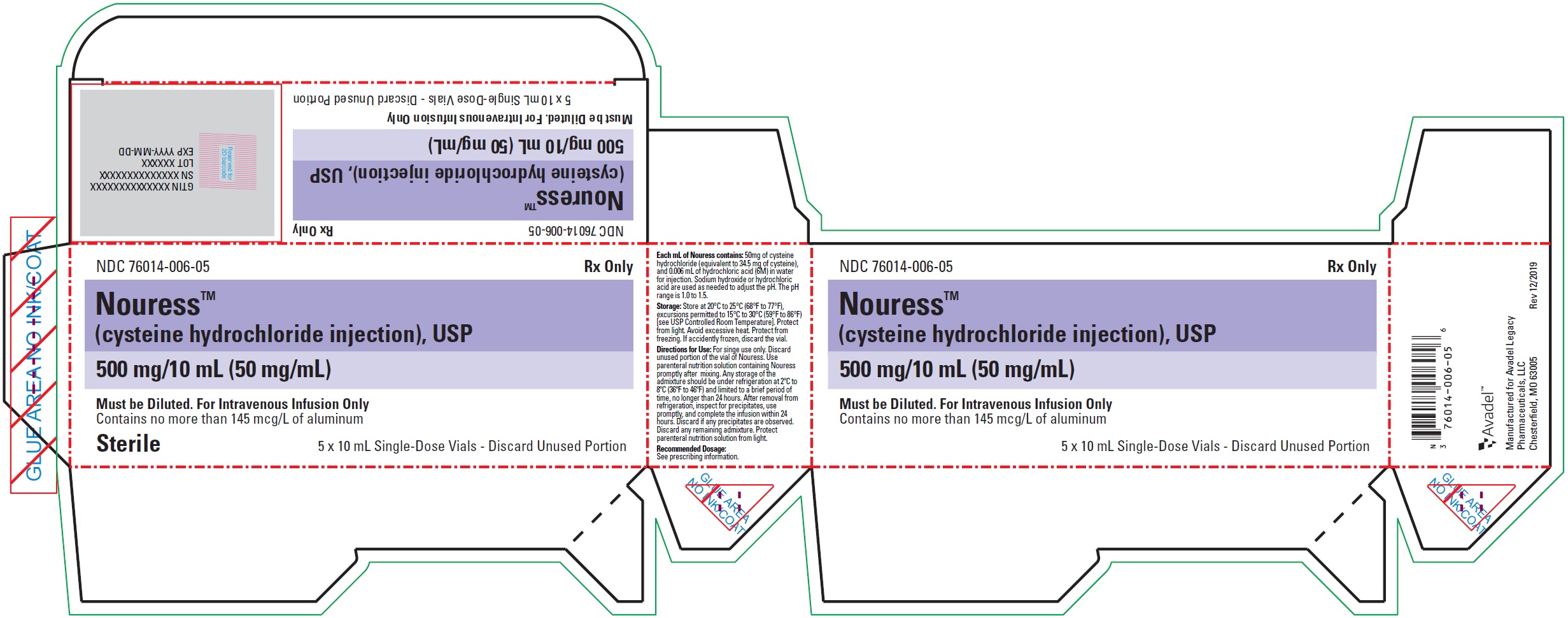
500 mg/10 mL (50 mg/mL) of cysteine hydrochloride, USP in single-dose vials (NDC: 76014-006-33), packaged as 5 vials per carton (NDC: 76014-006-05).
Store NOURESS at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Protect from light. Avoid excessive heat. Protect from freezing. If accidentally frozen, discard the vial.
Vial stoppers are not made with natural rubber latex.
For storage of admixed solution see Dosage and Administration (2.3).
-
17 PATIENT COUNSELING INFORMATION
Inform caregivers or home healthcare providers of the following risks of NOURESS:
- Pulmonary embolism due to pulmonary vascular precipitates [see Warnings and Precautions (5.1)]
- Vein damage and thrombosis [see Warnings and Precautions (5.2)]
- Increased BUN [see Warnings and Precautions (5.3)]
- Acid-base imbalance [see Warnings and Precautions (5.4)]
- Hepatobiliary disorders [see Warnings and Precautions (5.5)]
- Aluminum toxicity [see Warnings and Precautions (5.6)]
- Monitoring and laboratory tests [see Warnings and Precautions (5.7)]
Manufactured for:
Avadel Legacy Pharmaceuticals, LLC
Chesterfield, MO 63005
Rev. 12/19
- PRINCIPAL DISPLAY PANEL - NDC: 76014-006-33 - Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 76014-006-05 - Carton Label
-
INGREDIENTS AND APPEARANCE
NOURESS
cysteine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76014-006 Route of Administration Intravenous Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYSTEINE HYDROCHLORIDE (UNII: ZT934N0X4W) (CYSTEINE - UNII:K848JZ4886) CYSTEINE 34.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76014-006-05 5 in 1 CARTON 04/01/2020 1 NDC: 76014-006-33 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212535 04/01/2020 Labeler - Avadel Legacy Pharmaceuticals, LLC (965538205)
Trademark Results [Nouress]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NOURESS 88091317 not registered Live/Pending |
Flamel Ireland Limited 2018-08-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.