PENTAZOCINE HYDROCHLORIDE AND ACETAMINOPHEN tablet
Pentazocine Hydrochloride and Acetaminophen by
Drug Labeling and Warnings
Pentazocine Hydrochloride and Acetaminophen by is a Prescription medication manufactured, distributed, or labeled by GAVIS Pharmaceuticals, LLC, Novel Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
Pentazocine Hydrochloride and Acetaminophen Tablets contain acetaminophen and pentazocine hydrochloride. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
-
DESCRIPTION
Pentazocine Hydrochloride and Acetaminophen Tablets are a combination of pentazocine hydrochloride, USP, equivalent to 25 mg base and acetaminophen, USP, 650 mg.
Pentazocine is a member of the benzazocine series (also known as the benzomorphan series). Chemically, pentazocine is (2R*,6R*,11R*)1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol, a white, crystalline substance soluble in acidic aqueous solutions, and has the following structural formula:
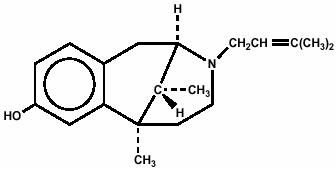
C19H27NO HCl M.W. 321.88
Chemically, acetaminophen is Acetamide, N-(4-hydroxyphenyl)-, and has the following structural formula:
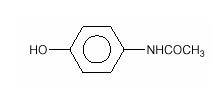
C8H9NO2 M.W. 151.16
Pentazocine is an analgesic and acetaminophen is an analgesic and antipyretic.
Inactive ingredients: colloidal silicon dioxide, FD&C Blue # 1 aluminum lake, microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate, and stearic acid.
-
CLINICAL PHARMACOLOGY
Pentazocine is a Schedule IV opioid analgesic with agonist/antagonist action which when administered orally is approximately equivalent on a mg for mg basis in analgesic effect to codeine.
Acetaminophen is an analgesic and antipyretic.
Pentazocine weakly antagonizes the analgesic effects of morphine, meperidine, and phenazocine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity.
Onset of significant analgesia with pentazocine usually occurs between 15 and 30 minutes after oral administration, and duration of action is usually three hours or longer.
Pentazocine is well absorbed from the gastrointestinal tract. Plasma levels closely correspond to the onset, duration, and intensity of analgesia. The time to mean peak concentration in 24 normal volunteers was 1.7 hours (range 0.5 to 4 hours) after oral administration and the mean plasma elimination half-life was 3.6 hours (range 1.5 to 10 hours).
The action of pentazocine is terminated for the most part by biotransformation in the liver with some free pentazocine excreted in the urine. The products of the oxidation of the terminal methyl groups and glucuronide conjugates are excreted by the kidney. Elimination of approximately 60% of the total dose occurs within 24 hours. Pentazocine passes into fetal circulation.
Onset of significant analgesic and antipyretic activity of acetaminophen when administered orally occurs within 30 minutes and is maximal at approximately 2 1/2 hours. The pharmacological mode of action of acetaminophen is unknown at this time.
Acetaminophen is rapidly and almost completely absorbed from the gastrointestinal tract. In 24 normal volunteers the time to mean peak plasma concentration was 1 hour (range 0.25 to 3 hours) after oral administration and the mean plasma elimination half-life was 2.8 hours (range 2 to 4 hours).
The effect of pentazocine on acetaminophen plasma protein binding or vice versa has not been established. For acetaminophen there is little or no plasma protein binding at normal therapeutic doses. When toxic doses of acetaminophen are ingested and drug plasma levels exceed 90 mcg/mL, plasma binding may vary from 8% to 43%.
Acetaminophen is conjugated in the liver with glucuronic acid and to a lesser extent with sulfuric acid. Approximately 80% of acetaminophen is excreted in the urine after conjugation and about 3% is excreted unchanged. The drug is also conjugated to a lesser extent with cysteine and additionally metabolized by hydroxylation.
If pentazocine hydrochloride and acetaminophen tablets are taken every 4 hours over an extended period of time, accumulation of pentazocine and to a lesser extent, acetaminophen, may occur.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hepatotoxicity
Pentazocine hydrochloride and acetaminophen tablets contain acetaminophen and pentazocine hydrochloride. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
Hypersensitivity/anaphylaxis
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergent medical attention. Instruct patients to discontinue pentazocine hydrochloride and acetaminophen tablets immediately and seek medical care if they experience these symptoms. Do not prescribe pentazocine hydrochloride and acetaminophen tablets for patients with acetaminophen allergy.
Drug Dependence
Pentazocine can cause a physical and psychological dependence. (See DRUG ABUSE AND DEPENDENCE.)
Use In Head Injury and Increased Intracranial Pressure
In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the possible respiratory depressant effects of pentazocine and its potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly increased. Furthermore, pentazocine can produce effects on pupillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries. In such patients, pentazocine hydrochloride and acetaminophen tablets must be used with extreme caution and only if its use is deemed essential.
Interactions with Alcohol and Drugs of Abuse
Pentazocine may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression because respiratory depression, hypotension, profound sedation, coma or death may result.
Patients Receiving Narcotics
Pentazocine is a mild narcotic antagonist. Some patients previously given narcotics, including methadone for the daily treatment of narcotic dependence, have experienced withdrawal symptoms after receiving pentazocine.
Respiratory Depression
Respiratory depression occurs more frequently in elderly or debilitated patients and in those suffering from conditions accompanied by hypoxia, hypercapnia, or upper airway obstruction, in whom even moderate therapeutic doses may significantly decrease pulmonary ventilation. Use pentazocine hydrochloride and acetaminophen tablets with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale and in patients having a substantially decreased respiratory reserve (e.g., severe kyphoscoliosis), hypoxia, hypercapnia, or pre-existing respiratory depression. Alternative non-opioid analgesics should be considered, and pentazocine hydrochloride and acetaminophen tablets should be employed only under careful medical supervision at the lowest effective dose in such patients.
Acute CNS Manifestations
Patients receiving therapeutic doses of pentazocine hydrochloride and acetaminophen tablets have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be closely observed and vital signs checked. If the drug is reinstituted, it should be done with caution since these acute CNS manifestations may recur.
-
PRECAUTIONS
Drug Abuse and Dependence
Pentazocine hydrochloride and acetaminophen tablets are a Schedule IV controlled substance.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of a drug for non-medical purposes, often in combination with other psychoactive substances. Addiction is a disease of repeated drug abuse. Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. Addiction is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by misuse of the drug for non-medical purposes, and often in combination with other psychoactive substances.
There have been some reports of dependence and of withdrawal symptoms with pentazocine hydrochloride and acetaminophen tablets. Patients with a history of drug dependence should be under close supervision while receiving pentazocine orally. There have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy.
There have been reports of development of addiction and physical dependence in patients receiving parenteral pentazocine. People with a history of drug abuse or alcohol abuse may have a higher chance of becoming addicted to opioid medicines.
Abrupt dose cessation or rapid dose reduction following the extended use of parenteral pentazocine has resulted in withdrawal symptoms such as abdominal cramps, nausea, vomiting, elevated temperature, chills, rhinorrhea, restlessness, anxiety, or lacrimation. In general opioid therapy should not be abruptly discontinued. When the patient no longer requires treatment with pentazocine hydrochloride and acetaminophen tablets, the drug should be tapered gradually to prevent signs and symptoms of withdrawal in patients who have been receiving opioids for an extended period of time and might have become physically dependent.
In prescribing pentazocine hydrochloride and acetaminophen tablets for chronic use, the physician should take under consideration that proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to identify and decrease misuse and abuse of opioid drugs.
Severe, even lethal, consequences may result from misuse of tablets by injection either alone or in combination with other substances, such as pulmonary emboli, vascular occlusion, ulceration and abscesses, and withdrawal symptoms in narcotic dependent individuals.
CNS Effect
Caution should be used when pentazocine hydrochloride and acetaminophen tablets are administered to patients prone to seizures; seizures have occurred in a few such patients in association with the use of pentazocine although no cause and effect relationship has been established.
Porphyria
Particular caution should be exercised in administering pentazocine to patients with porphyria since it may provoke an acute attack in susceptible individuals.
Cardiovascular Disease
Pentazocine can elevate blood pressure, possibly through the release of endogenous catecholamines. Particular caution should be exercised in conditions where alterations in vascular resistance and blood pressure might be particularly undesirable, such as in the acute phase of myocardial infarction.
Pentazocine hydrochloride and acetaminophen tablets should be used with caution in patients with myocardial infarction who have nausea or vomiting.
Impaired Renal or Hepatic Function
Decreased metabolism of the drug by the liver in extensive liver disease may predispose to accentuation of side effects. Although laboratory tests have not indicated that pentazocine causes or increases renal or hepatic impairment, the drug should be administered with caution to patients with such impairment.
Acetaminophen is metabolized by the liver, and has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Therefore, pentazocine hydrochloride and acetaminophen tablets should be administered with caution to patients with hepatic impairment and in individuals who ingest alcohol (See BOXED WARNING and WARNINGS, Hepatotoxicity).
Other
Caution should also be observed when administering pentazocine hydrochloride and acetaminophen tablets in patients with hypothyroidism, adrenocortical insufficiency, prostate hypertrophy, inflammatory or obstructive bowel disease, acute abdominal syndromes of unknown etiology, cholecystitis, pancreatitis, or acute alcohol intoxication and delirium tremens.
Biliary Surgery
Narcotic drug products are generally considered to elevate biliary tract pressure for varying periods following their administration. Some evidence suggests that pentazocine may differ from other marketed narcotics in this respect (i.e., it causes little or no elevation in biliary tract pressures). The clinical significance of these findings, however, is not yet known.
Information for Patients
Patients receiving pentazocine hydrochloride and acetaminophen tablets should be given the following instructions by the physician:
- Do not take pentazocine hydrochloride and acetaminophen tablets if you are allergic to any of its ingredients.
- If you develop signs/symptoms of allergy such as a rash, hives, itching, vomiting, swelling of the face or mouth, or difficulty breathing, stop taking pentazocine hydrochloride and acetaminophen tablets and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen (alone or in combination with other products containing acetaminophen). Seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if you feel well. Look for acetaminophen or APAP on package labels. Do not use more than one product that contains acetaminophen.
- Contact your healthcare provider if you took more than the recommended dose.
- Patients should be advised that pentazocine hydrochloride and acetaminophen tablet is a narcotic pain reliever, and should be taken only as directed.
- The dose of pentazocine hydrochloride and acetaminophen tablets should not be adjusted without consulting with a physician or other healthcare professional.
- Patients should be advised that pentazocine hydrochloride and acetaminophen tablets may cause drowsiness, dizziness, or lightheadedness and may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating machinery). Patients started on pentazocine hydrochloride and acetaminophen tablets or patients whose dose has been adjusted should refrain from any potentially dangerous activity until it is established that they are not adversely affected.
- Pentazocine hydrochloride and acetaminophen tablets will add to the effect of alcohol and other CNS depressants (such as antihistamines, sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and monoamine oxidase [MAO]inhibitors).
- Patients should not combine pentazocine hydrochloride and acetaminophen tablets with alcohol or other central nervous system depressants (sleep aids, tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death.
- Women of childbearing potential who become or are planning to become pregnant should consult a physician prior to initiating or continuing therapy with pentazocine hydrochloride and acetaminophen tablets.
- Safe use in pregnancy has not been established. Prolonged use of opioid analgesics during pregnancy may cause neonatal physical dependence, and neonatal withdrawal may occur.
- If patients have been receiving treatment with pentazocine hydrochloride and acetaminophen tablets for more than a few weeks and cessation of therapy is indicated, they should be counseled on the importance of safely tapering the dose and that abruptly discontinuing the medication could precipitate withdrawal symptoms. The physician should provide a dose schedule to accomplish a gradual discontinuation of the medication.
- Patients should be advised that pentazocine hydrochloride and acetaminophen tablets are a potential drug of abuse. They should protect it from theft. It should never be given to anyone other than the individual for whom it was prescribed.
- Patients should be instructed to keep pentazocine hydrochloride and acetaminophen tablets in a secure place out of the reach of children. When pentazocine hydrochloride and acetaminophen tablets are no longer needed, please consult your pharmacist for proper disposal instructions.
- As with other opioids, patients taking pentazocine hydrochloride and acetaminophen tablets should be advised of the potential for severe constipation; appropriate laxatives and/or stool softeners as well as other appropriate treatments should be initiated from the onset of opioid therapy.
- Patients should be advised of the most common adverse events that may occur while taking pentazocine hydrochloride and acetaminophen tablets: constipation, nausea, somnolence, lightheadedness, dizziness, sedation, vomiting, and sweating.
Drug Interactions
CNS Depressants
Other central nervous system (CNS) depressants including sedatives, hypnotics, general anesthetics, antiemetics, phenothiazines, or other tranquilizers or alcohol increases the risk of respiratory depression, hypotension, profound sedation, or coma. Use morphine sulfate with caution and in reduced dosages in patients taking these agents.
Opioid Agonist Analgesics
Pentazocine hydrochloride and acetaminophen tablets can antagonize the effects of a pure opioid agonist analgesic and/or may precipitate withdrawal symptoms.
Monoamine Oxidase Inhibitors (MAOIs)
Concomitant use of monoamine oxidase inhibitors (MAOIs) with pentazocine hydrochloride and acetaminophen tablets may cause CNS excitation and hypertension through their respective effects on catecholamines. Caution should therefore be observed in administering pentazocine hydrochloride and acetaminophen tablets to patients who are currently receiving MAOIs or who have received them within the preceding 14 days.
Anticholinergics
Anticholinergics or other medications with anticholinergic activity when used concurrently with opioid analgesics may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
Tobacco
Smoking tobacco could enhance the metabolic clearance rate of pentazocine reducing the clinical effectiveness of a standard dose of pentazocine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis, mutagenesis, and impairment of fertility studies have not been done with this combination product.
Studies to evaluate the mutagenic potential of the components of pentazocine hydrochloride and acetaminophen tablets have not been conducted.
Pentazocine, when administered orally or parenterally, had no adverse effect on either the reproductive capabilities or the course of pregnancy in rabbits and rats. Embryotoxic effects on the fetuses were not shown.
The daily administration of 4 mg/kg to 20 mg/kg pentazocine subcutaneously to female rats during a 14 day pre-mating period and until the 13th day of pregnancy did not have any adverse effects on the fertility rate.
Pregnancy
Teratogenic Effects
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Pentazocine hydrochloride and acetaminophen tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal studies with the combination of pentazocine and acetaminophen have not been completed.
In a published report, a single dose of pentazocine administered to pregnant hamsters on gestation day 8 increased the incidence of exencephaly and cranioschisis at a dose of 196 mg/kg, SC (0.2-times the maximum daily human dose of pentazocine via 6 caplets on a mg/m2 basis).
Nonteratogenic Effects
There has been no experience in this regard with the combination pentazocine and acetaminophen. However, there have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy. Frequent use of acetaminophen (defined as most days or daily use) in late pregnancy may be associated with an increased risk of persistent wheezing in the infant which may persist into childhood.
Labor and Delivery
Patients receiving pentazocine during labor have experienced no adverse effects other than those that occur with commonly used analgesics. However, pentazocine can cross the placental barrier and cause central nervous system depression in the newborn and, if used regularly throughout pregnancy, may lead to symptoms of withdrawal in the newborn. Pentazocine hydrochloride and acetaminophen tablets should be used with caution in women delivering premature infants. The effect of pentazocine hydrochloride and acetaminophen tablets on the mother and fetus, the duration of labor or delivery, the possibility that forceps delivery or other intervention or resuscitation of the newborn may be necessary, or the effect of pentazocine hydrochloride and acetaminophen tablets, on the later growth, development, and functional maturation of the child are unknown at the present time.
Nursing Mothers
Pentazocine and acetaminophen are excreted in human milk. Caution should be exercised when pentazocine hydrochloride and acetaminophen tablets are administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Geriatric Use
Clinical studies of pentazocine hydrochloride and acetaminophen tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Clinical experience with pentazocine hydrochloride and acetaminophen tablets have been insufficient to define all possible adverse reactions with this combination. However, reactions reported after oral administration of pentazocine hydrochloride in 50 mg dosage include the following:
Cardiovascular: hypertension, hypotension, circulatory depression, tachycardia.
Respiratory: rarely respiratory depression,
Acute CNS Manifestations: Hallucinations (usually visual), disorientation, and confusion
Other CNS effects: grand mal convulsions, increase in intracranial pressure, dizziness, lightheadedness, hallucinations, sedation, euphoria, headache, confusion, disorientation; infrequently weakness, disturbed dreams, insomnia, syncope, and depression; and rarely tremor, irritability, excitement, tinnitus.
Autonomic: sweating; infrequently flushing; and rarely chills.
Gastrointestinal: nausea, vomiting, constipation; diarrhea, anorexia, dry mouth, Biliary tract spasm, and rarely abdominal distress.
Allergic: edema of the face, anaphylactic shock, dermatitis including pruritus, flushed skin including plethora, infrequently rash; and rarely urticaria.
Ophthalmic: visual blurring and focusing difficulty, miosis.
Hematologic: depression of white blood cells (especially granulocytes) with rare cases of agranulocytosis, which is usually reversible, moderate transient eosinophilia.
Dependence and Withdrawal Symptoms: (See WARNINGS, PRECAUTIONS, and DRUG ABUSE AND DEPENDENCE Sections).
Other: urinary retention, paresthesia, serious skin reactions, including erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis, and alterations in rate or strength of uterine contractions during labor.
A few cases of hypersensitivity to acetaminophen have been reported, as manifested by anaphylaxis, angioneurotic edema, thrombocytopenic purpura, skin rashes, and rarely hemolytic anemia and agranulocytosis. Occasionally individuals respond to ordinary doses with nausea and vomiting and diarrhea.
-
OVERDOSAGE
Signs and Symptoms
For pentazocine alone in single doses above 60 mg there have been reports of the occurrence of nalorphine-like psychotomimetic effects such as anxiety, nightmares, strange thoughts, and hallucinations. Somnolence, marked respiratory depression associated with increased blood pressure and tachycardia have also resulted as have seizures, hypotension, dizziness, nausea, vomiting, lethargy, and paresthesias. The respiratory depression is antagonized by naloxone (see Treatment). Circulatory failure and deepening coma may occur in more severe cases, particularly in patients who have also ingested other CNS depressants such as alcohol, sedative/hypnotics, or antihistamines.
In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and coagulation defects may also occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48-72 hours post-ingestion.
Treatment
Adequate measures to maintain ventilation and general circulatory support should be employed. Assisted or controlled ventilation, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Consideration should be given to gastric lavage and gastric aspiration to reduce drug absorption.
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered. For respiratory depression due to overdosage or unusual sensitivity to pentazocine hydrochloride and acetaminophen tablets, parenteral naloxone is a specific and effective antagonist.
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less then 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
-
DOSAGE AND ADMINISTRATION
Adult
The usual adult dose is 1 caplet every 4 hours as needed for pain relief, up to a maximum of 6 caplets per day.
Discontinuation
Due to the potential for withdrawal symptoms associated with abrupt discontinuation, consideration should be given to tapering patients off pentazocine hydrochloride and acetaminophen tablets after prolonged periods of treatment with pentazocine hydrochloride and acetaminophen tablets (See PRECAUTIONS, Drug Abuse and Dependence).
-
HOW SUPPLIED
Pentazocine Hydrochloride and Acetaminophen Tablets are light blue capsule shaped, tablets debossed “NL” on left side and “670” on right side of bisect and plain on the other side.
Bottles of 100 (NDC: 43386-670-01).
Bottles of 100 (NDC: 43386-670-05)
Store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F). [See USP.]
Dispense in a tight, light-resistant container as defined in the USP.
DEA Order Form Required.
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873Distributed by:
GAVIS Pharmaceuticals, LLC
Somerset, NJ 08873GIN-670-02
Rev: 06/2011 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PENTAZOCINE HYDROCHLORIDE AND ACETAMINOPHEN
pentazocine hydrochloride and acetaminophen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 43386-670 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PENTAZOCINE HYDROCHLORIDE (UNII: A36BXO4PPX) (PENTAZOCINE - UNII:RP4A60D26L) PENTAZOCINE HYDROCHLORIDE 25 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color BLUE Score 2 pieces Shape CAPSULE Size 19mm Flavor Imprint Code NL;670 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43386-670-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076202 05/11/2011 Labeler - GAVIS Pharmaceuticals, LLC (829838551) Establishment Name Address ID/FEI Business Operations Novel Laboratories, Inc. 793518643 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
