ATMEKSI- methocarbamol suspension
ATMEKSI by
Drug Labeling and Warnings
ATMEKSI by is a Prescription medication manufactured, distributed, or labeled by Metacel Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ATMEKSI® (methocarbamol) oral suspension safely and effectively. See full prescribing information for ATMEKSI (methocarbamol) oral suspension.
ATMEKSI (methocarbamol) oral suspension
Initial U.S. Approval: 05/07/2018INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Adults
Initial dosage: 1,500 mg (10 mL) 4 times daily
Maintenance dosage: 750 mg (5 mL) every 4 hours or 1,500 mg (10 mL) 3 times daily
Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered). Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
DOSAGE FORMS AND STRENGTHS
- oral suspension: 750 mg/5 mL
CONTRAINDICATIONS
Hypersensitivity to ATMEKSI or any component in the product ( 4.1)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- Body:Anaphylactic reaction, angioneurotic edema, fever, headache ( 6)
- Cardiovascular system:Bradycardia, flushing, hypotension, syncope, thrombophlebitis ( 6)
- Digestive system:Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting ( 6)
- Hemic and lymphatic system:Leukopenia ( 6)
- Immune system:Hypersensitivity reactions ( 6)
- Nervous system:Amnesia, confusion, diplopia, dizziness or lightheadedness, drowsiness, insomnia, mild muscular incoordination, nystagmus, sedation, seizures (including grand mal), vertigo ( 6)
- Skin and special senses:Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash and urticaria ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Rosemont Pharmaceuticals, LLC. at 1-844-638-2235 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
DRUG INTERACTIONS
- ATMEKSI may inhibit the effect of pyridostigmine bromide. Patients with myasthenia gravis should be monitored closely for symptoms of myasthenia gravis such as weakness. If symptoms of myasthenia gravis are observed, treatment with ATMEKSI should be stopped immediately ( 7.2)
- Laboratory test interference: Methocarbamol may cause color interference in the following screening tests: 5-hydroxyindoleacetic acid (using nitrosonaphthol reagent) and VMA (Giltow method) ( 7.3)
USE IN SPECIFIC POPULATIONS
Revised: 8/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acute, painful musculoskeletal conditions.
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 CNS Depressants and Alcohol Use
5.2 Use in Activities Requiring Mental Alertness
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 CNS drugs and alcohol
7.2 Pyridostigmine Bromide
7.3 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Initial dosage: 1,500 mg (10 mL) 4 times daily
Maintenance dosage: 750 mg (5 mL) every 4 hours or 1,500 mg (10 mL) 3 times daily
Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered). Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
Inform the patient to shake the drug product for at least 30 seconds to ensure it is uniform before administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 CNS Depressants and Alcohol Use
ATMEKSI may potentiate the effects of CNS (central nervous system) depressants and alcohol. Patients receiving ATMEKSI (methocarbamol) Oral Suspension should be cautioned about combined effects with alcohol and other CNS depressants [ see Drug Interactions (7.1)].
5.2 Use in Activities Requiring Mental Alertness
ATMEKSI may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
-
6 ADVERSE REACTIONS
The following serious adverse reaction is described elsewhere in the labeling:
- Interactions with CNS Depressants and Alcohol [ see Warnings and Precautions (5.1)]
The following adverse reactions associated with the use of methocarbamol have been identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported with the administration of methocarbamol include:
Body as a whole: Anaphylactic reaction, angioneurotic edema, fever, headache.
Cardiovascular system:Bradycardia, flushing, hypotension, syncope, thrombophlebitis.
Digestive system:Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting.
Hemic and lymphatic system:Leukopenia.
Immune System:Hypersensitivity reactions.
Nervous system:Amnesia, confusion, diplopia, dizziness or lightheadedness, drowsiness, insomnia, mild muscular incoordination, nystagmus, sedation, seizures (including grand mal), vertigo.
Skin and special senses:Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash, urticaria.
-
7 DRUG INTERACTIONS
7.1 CNS drugs and alcohol
ATMEKSI may potentiate the effects of CNS (central nervous system) depressants and alcohol [ see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data from case reports over decades of use with methocarbamol during pregnancy have not identified an increased risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized patients is 2 to 4% and 15 to 20% respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of methocarbamol or its metabolites in human milk, the effects on a breastfed infant or the effects on milk production. Methocarbamol and/or its metabolite are present in animal milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ATMEKSI and any potential adverse effects on the breastfed infant from ATMEKSI or the underlying maternal condition.
-
10 OVERDOSAGE
Clinical Presentation
Deaths have been reported with an overdose of methocarbamol alone and when methocarbamol was used with other CNS depressants, including alcohol. Limited information is available on the acute toxicity of methocarbamol. Symptoms of overdose include the following: respiratory depression, nausea, drowsiness, blurred vision, hypotension, seizures, coma, and death. Other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants) can have an additive effect, even when taken at recommended dosages.
Treatment of Overdose
The standard of treatment is supportive care, including monitoring for CNS and respiratory depression and managing airway as needed, monitoring urinary output and vital signs, and administration of intravenous fluids if necessary.
Hypotension should be treated with intravenous fluids and vasopressors as needed. Gastrointestinal decontamination procedures (including emesis) should generally be avoided because aspiration may result from CNS depression and seizures. Extracorporeal elimination such as hemodialysis or plasmapheresis has no proven clinical benefit. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
ATMEKSI (methocarbamol) Oral Suspension is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.
The chemical name of methocarbamol is (±)1,2-Propanediol, 3-(2-methoxyphenoxy)-, 1-carbamate, or (±)-3-(o-Methoxyphenoxy)-1,2-propanediol 1-carbamate and has the empirical formula C11H15NO5. Its molecular weight is 241.24g/mol. The structural formula is shown below:
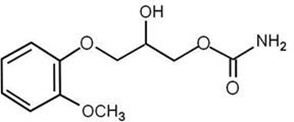
Methocarbamol is a white powder, sparingly soluble in water and in chloroform, soluble in alcohol (only with heating), insoluble in benzene and in n-hexane.
ATMEKSI (methocarbamol) Oral Suspension is a white to off-white suspension with a fruit flavor and a pH range of 3.2 – 4.8.
ATMEKSI (methocarbamol) Oral Suspension contains the following inactive ingredients: citric acid monohydrate, glycerin, magnesium aluminum silicate, purified water, sodium benzoate, sodium carboxymethylcellulose, sodium citrate, sucralose and fruit flavor.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of methocarbamol in humans has not been established but may be due to general central nervous system (CNS) depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate, or the nerve fiber.
12.3 Pharmacokinetics
Absorption
After oral administration in healthy volunteers, ATMEKSI is rapidly absorbed with a median Tmax of 0.66 hours. Food decreases the extent and delays the rate of absorption of methocarbamol. In the presence of food, the Cmax is decreased by 51% and the AUC is decreased by 31%. The median Tmax is delayed to 1.6 hours.
Distribution
Methocarbamol demonstrates moderate binding to plasma proteins, typically ranging from 46% to 50%.
Elimination
In healthy volunteers, the plasma clearance of methocarbamol ranges between 0.20 and 0.80 L/h/kg. The mean plasma elimination half-life of ATMEKSI in healthy volunteers is approximately 1.3 hours and 1.5 hours when administered with or without food.
Metabolism
Methocarbamol is metabolized via dealkylation and hydroxylation. Conjugation of methocarbamol also is likely. Essentially all methocarbamol metabolites are eliminated in the urine.
Excretion
Small amounts of unchanged methocarbamol also are excreted in the urine.
Specific Populations
Geriatric Patients
The mean (± SD) elimination half-life of methocarbamol in elderly healthy volunteers (mean (± SD) age, 69 (± 4) years) was slightly prolonged compared to a younger (mean (± SD) age, 53.3 (± 8.8) years), healthy population (1.5 (± 0.4) hours versus 1.1 (± 0.27) hours, respectively). The fraction of bound methocarbamol was slightly decreased in the elderly versus younger volunteers (41 to 43% versus 46 to 50%, respectively).
Pediatric Patients
Safety and effectiveness of methocarbamol oral suspension in pediatric patients below the age of 16 have not been established.
Patients with Renal Impairment
The clearance of methocarbamol in 8 renally-impaired patients on maintenance hemodialysis was reduced about 40% compared to 17 normal subjects, although the mean (± SD) elimination half-life in these two groups was similar: 1.2 (± 0.6) versus 1.1 (± 0.3) hours, respectively.
Patients with Hepatic Impairment
In 8 patients with cirrhosis secondary to alcohol abuse, the mean total clearance of methocarbamol was reduced approximately 70% compared to that obtained in 8 age- and weight-matched normal subjects. The mean (± SD) elimination half-life in the cirrhotic patients and the normal subjects was 3.38 (± 1.62) hours and 1.11 (± 0.27) hours, respectively. The percent of methocarbamol bound to plasma proteins was decreased to approximately 40 to 45% compared to 46 to 50% in the normal subjects.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed.
Mutagenesis
No studies have been conducted to assess the effect of methocarbamol on mutagenesis.
Impairment of Fertility
No studies have been conducted to assess the effect of methocarbamol on its potential to impair fertility.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ATMEKSI (methocarbamol) Oral Suspension contains 750 mg/5 mL methocarbamol. It is a white to off-white suspension with a fruit flavor and is supplied in bottles of 150 mL with a child-resistant closure (NDC: 69528-701-05).
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
- Principal Display Panel - 150 mL Bottle
-
INGREDIENTS AND APPEARANCE
ATMEKSI
methocarbamol suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69528-701 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOCARBAMOL (UNII: 125OD7737X) (METHOCARBAMOL - UNII:125OD7737X) METHOCARBAMOL 750 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERIN (UNII: PDC6A3C0OX) SUCRALOSE (UNII: 96K6UQ3ZD4) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69528-701-05 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219843 12/22/2025 Labeler - Metacel Pharmaceuticals, LLC (079475312)
Trademark Results [ATMEKSI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ATMEKSI 98200219 not registered Live/Pending |
Rosemont Pharmaceuticals Limited 2023-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
