SALAGEN- pilocarpine hydrochloride tablet, film coated
Salagen by
Drug Labeling and Warnings
Salagen by is a Prescription medication manufactured, distributed, or labeled by Concordia Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
SALAGEN® Tablets contain pilocarpine hydrochloride, a cholinergic agonist for oral use. Pilocarpine hydrochloride is a hygroscopic, odorless, bitter tasting white crystal or powder which is soluble in water and alcohol and virtually insoluble in most non-polar solvents. Pilocarpine hydrochloride, with a chemical name of (3S-cis)-2(3H)-Furanone, 3-ethyl-dihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl] mono-hydrochloride, has a molecular weight of 244.72.
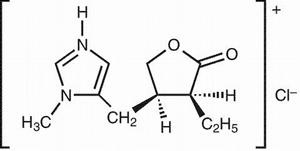
Each 5 mg SALAGEN® Tablet for oral administration contains 5 mg of pilocarpine hydrochloride. Inactive ingredients in the tablet, the tablet’s film coating, and polishing are: carnauba wax, hypromellose, microcrystalline cellulose, stearic acid, titanium dioxide and other ingredients.
Each 7.5 mg SALAGEN® Tablet for oral administration contains 7.5 mg of pilocarpine hydrochloride. Inactive ingredients in the tablet, the tablet’s film coating, and polishing are: carnauba wax, hypromellose, microcrystalline cellulose, stearic acid, titanium dioxide, FD&C blue#2 aluminum lake, and other ingredients. -
CLINICAL PHARMACOLOGY
Pharmacodynamics:
Pilocarpine is a cholinergic parasympathomimetic agent exerting a broad spectrum of pharmacologic effects with predominant muscarinic action. Pilocarpine, in appropriate dosage, can increase secretion by the exocrine glands. The sweat, salivary, lacrimal, gastric, pancreatic, and intestinal glands and the mucous cells of the respiratory tract may be stimulated. When applied topically to the eye as a single dose it causes miosis, spasm of accommodation, and may cause a transitory rise in intraocular pressure followed by a more persistent fall. Dose-related smooth muscle stimulation of the intestinal tract may cause increased tone, increased motility, spasm, and tenesmus. Bronchial smooth muscle tone may increase. The tone and motility of urinary tract, gallbladder, and biliary duct smooth muscle may be enhanced. Pilocarpine may have paradoxical effects on the cardiovascular system. The expected effect of a muscarinic agonist is vasodepression, but administration of pilocarpine may produce hypertension after a brief episode of hypotension. Bradycardia and tachycardia have both been reported with use of pilocarpine.
In a study of 12 healthy male volunteers there was a dose-related increase in unstimulated salivary flow following single 5 and 10 mg oral doses of SALAGEN® Tablets. This effect of pilocarpine on salivary flow was time-related with an onset at 20 minutes and a peak effect at 1 hour with a duration of 3 to 5 hours (See Pharmacokinetics section).
Head & Neck Cancer Patients: In a 12 week randomized, double-blind, placebo-controlled study in 207 patients (placebo, N=65; 5 mg, N=73; 10 mg, N=69), increases from baseline (means 0.072 and 0.112 mL/min, ranges -0.690 to 0.728 and -0.380 to 1.689) of whole saliva flow for the 5 mg (63%) and 10 mg (90%) tablet, respectively, were seen 1 hour after the first dose of SALAGEN® Tablets. Increases in unstimulated parotid flow were seen following the first dose (means 0.025 and 0.046 mL/min, ranges 0 to 0.414 and -0.070 to 1.002 mL/min for the 5 and 10 mg dose, respectively). In this study, no correlation existed between the amount of increase in salivary flow and the degree of symptomatic relief.
Sjogren's Syndrome Patients: In two 12 week randomized, double-blind, placebo-controlled studies in 629 patients (placebo, N=253; 2.5 mg, N=121; 5 mg, N=255; 5-7.5 mg, N=114), the ability of SALAGEN® Tablets to stimulate saliva production was assessed. In these trials using varying doses of SALAGEN® Tablets (2.5-7.5 mg), the rate of saliva production was plotted against time. An Area Under the Curve (AUC) representing the total amount of saliva produced during the observation interval was calculated. Relative to placebo, an increase in the amount of saliva being produced was observed following the first dose of SALAGEN® Tablets and was maintained throughout the duration (12 weeks) of the trials in an approximate dose response fashion (See Clinical Studies section).Pharmacokinetics:
In a multiple-dose pharmacokinetic study in male volunteers following 2 days of 5 or 10 mg of oral pilocarpine hydrochloride tablets given at 8 a.m., noontime, and 6 p.m., the mean elimination half-life was 0.76 hours for the 5 mg dose and 1.35 hours for the 10 mg dose. Tmax values were 1.25 hours and 0.85 hours. Cmax values were 15 ng/mL and 41 ng/mL. The AUC trapezoidal values were 33 h(ng/mL) and 108 h(ng/mL), respectively, for the 5 and 10 mg doses following the last 6 hour dose.
Pharmacokinetics in elderly male volunteers (N=11) were comparable to those in younger men. In five healthy elderly female volunteers, the mean Cmax and AUC were approximately twice that of elderly males and young normal male volunteers.
When taken with a high fat meal by 12 healthy male volunteers, there was a decrease in the rate of absorption of pilocarpine from SALAGEN® Tablets. Mean Tmax's were 1.47 and 0.87 hours, and mean Cmax's were 51.8 and 59.2 ng/mL for fed and fasted, respectively.
Limited information is available about the metabolism and elimination of pilocarpine in humans. Inactivation of pilocarpine is thought to occur at neuronal synapses and probably in plasma. Pilocarpine and its minimally active or inactive degradation products, including pilocarpic acid, are excreted in the urine. Pilocarpine does not bind to human or rat plasma proteins over a concentration range of 5 to 25,000 ng/mL. The effect of pilocarpine on plasma protein binding of other drugs has not been evaluated.
In patients with mild to moderate hepatic impairment (N=12), administration of a single 5 mg dose resulted in a 30% decrease in total plasma clearance and a doubling of exposure (as measured by AUC). Peak plasma levels were also increased by about 30% and half-life was increased to 2.1 hrs.
There were no significant differences in the pharmacokinetics of oral pilocarpine in volunteer subjects (N=8) with renal insufficiency (mean creatinine clearances 25.4 mL/min; range 9.8 - 40.8 mL/min) compared to the pharmacokinetics previously observed in normal volunteers. -
CLINICAL STUDIES
Head & Neck Cancer Patients: A 12 week randomized, double-blind, placebo-controlled study in 207 patients (142 men, 65 women) was conducted in patients whose mean age was 58.5 years with a range of 19 to 77; the racial distribution was Caucasian 95%, Black 4%, and other 1%. In this population, a statistically significant improvement in mouth dryness occurred in the 5 and 10 mg SALAGEN® Tablet treated patients compared to placebo treated patients. The 5 and 10 mg treated patients could not be distinguished. (See Pharmacodynamics section for flow study details.)
Another 12 week, double-blind, randomized, placebo-controlled study was conducted in 162 patients whose mean age was 57.8 years with a range of 27 to 80; the racial distribution was Caucasian 88%, Black 10%, and other 2%. The effects of placebo were compared to 2.5 mg three times a day of SALAGEN® Tablets for 4 weeks followed by adjustment to 5 mg three times a day and 10 mg three times a day. Lowering of the dose was necessary because of adverse events in 3 of 67 patients treated with 5 mg of SALAGEN® Tablets and in 7 of 66 patients treated with 10 mg of SALAGEN® Tablets. After 4 weeks of treatment, 2.5 mg of SALAGEN® Tablets three times a day was comparable to placebo in relieving dryness. In patients treated with 5 mg and 10 mg of SALAGEN® Tablets, the greatest improvement in dryness was noted in patients with no measurable salivary flow at baseline.
In both studies, some patients noted improvement in the global assessment of their dry mouth, speaking without liquids, and a reduced need for supplemental oral comfort agents.
In the two placebo-controlled clinical trials, the most common adverse events related to drug, and increasing in rate as dose increases, were sweating, nausea, rhinitis, diarrhea, chills, flushing, urinary frequency, dizziness, and asthenia. The most common adverse experience causing withdrawal from treatment was sweating (5 mg t.i.d. ≤1%; 10 mg t.i.d. =12%).
Sjogren's Syndrome Patients: Two separate studies were conducted in patients with primary or secondary Sjogren's Syndrome. In both studies, the majority of patients best fit the European criteria for having primary Sjogren's Syndrome. ["Criteria for the Classification of Sjogren's Syndrome" (Vitali C, Bombardieri S, Moutsopoulos HM, et al: Preliminary criteria for the classification of Sjogren's Syndrome. Arthritis Rheum. 1993; 36:340-347.)]
A 12-week, randomized, double-blind, parallel-group, placebo-controlled study was conducted in 256 patients (14 men, 242 women) whose mean age was 57 years with a range of 24 to 85 years. The racial distribution was as follows: Caucasian 91%, Black 6%, and other 3%.
The effects of placebo were compared with those of SALAGEN® Tablets 5 mg four times a day (20 mg/day) for 6 weeks. At 6 weeks, the patients' dosage was increased from 5 mg SALAGEN® Tablets q.i.d. to 7.5 mg q.i.d. The data collected during the first 6 weeks of the trial were evaluated for safety and efficacy, and the data of the second 6 weeks of the trial were used to provide additional evidence of safety.
After 6 weeks of treatment, statistically significant global improvement of dry mouth was observed compared to placebo. "Global improvement" is defined as a score of 55 mm or more on a 100 mm visual analogue scale in response to the question, "Please rate your present condition of dry mouth (xerostomia) compared with your condition at the start of this study. Consider the changes to your dry mouth and other symptoms related to your dry mouth that have occurred since you have taken this medication." Patients' assessments of specific dry mouth symptoms such as severity of dry mouth, mouth discomfort, ability to speak without water, ability to sleep without drinking water, ability to swallow food without drinking, and a decreased use of saliva substitutes were found to be consistent with the significant global improvement described.
Another 12 week randomized, double-blind, parallel-group, placebo-controlled study was conducted in 373 patients (16 men, 357 women) whose mean age was 55 years with a range of 21 to 84. The racial distribution was Caucasian 80%, Oriental 14%, Black 2%, and 4% of other origin. The treatment groups were 2.5 mg pilocarpine tablets, 5 mg SALAGEN® Tablets, and placebo. All treatments were administered on a four times a day regimen.
After 12 weeks of treatment, statistically significant global improvement of dry mouth was observed at a dose of 5 mg compared with placebo. The 2.5 mg (10 mg/day) group was not significantly different than placebo. However, a subgroup of patients with rheumatoid arthritis tended to improve in global assessments at both the 2.5 mg q.i.d. (9 patients) and 5 mg q.i.d. (16 patients) dose (10-20 mg/day). The clinical significance of this finding is unknown.
Patients' assessments of specific dry mouth symptoms such as severity of dry mouth, mouth discomfort, ability to sleep without drinking water, and decreased use of saliva substitutes were also found to be consistent with the significant global improvement described when measured after 6 weeks and 12 weeks of SALAGEN® Tablets use. - INDICATIONS & USAGE
- CONTRAINDICATIONS
-
WARNINGS
Cardiovascular Disease: Patients with significant cardiovascular disease may be unable to compensate for transient changes in hemodynamics or rhythm induced by pilocarpine. Pulmonary edema has been reported as a complication of pilocarpine toxicity from high ocular doses given for acute angle-closure glaucoma. Pilocarpine should be administered with caution in and under close medical supervision of patients with significant cardiovascular disease.
Ocular: Ocular formulations of pilocarpine have been reported to cause visual blurring which may result in decreased visual acuity, especially at night and in patients with central lens changes, and to cause impairment of depth perception. Caution should be advised while driving at night or performing hazardous activities in reduced lighting.
Pulmonary Disease: Pilocarpine has been reported to increase airway resistance, bronchial smooth muscle tone, and bronchial secretions. Pilocarpine hydrochloride should be administered with caution to and under close medical supervision in patients with controlled asthma, chronic bronchitis, or chronic obstructive pulmonary disease requiring pharmacotherapy. -
PRECAUTIONS
General
Pilocarpine toxicity is characterized by an exaggeration of its parasympathomimetic effects. These may include: headache, visual disturbance, lacrimation, sweating, respiratory distress, gastrointestinal spasm, nausea, vomiting, diarrhea, atrioventricular block, tachycardia, bradycardia, hypotension, hypertension, shock, mental confusion, cardiac arrhythmia, and tremors.
The dose-related cardiovascular pharmacologic effects of pilocarpine include hypotension, hypertension, bradycardia, and tachycardia.
Pilocarpine should be administered with caution to patients with known or suspected cholelithiasis or biliary tract disease. Contractions of the gallbladder or biliary smooth muscle could precipitate complications including cholecystitis, cholangitis, and biliary obstruction.
Pilocarpine may increase ureteral smooth muscle tone and could theoretically precipitate renal colic (or "ureteral reflux"), particularly in patients with nephrolithiasis.
Cholinergic agonists may have dose-related central nervous system effects. This should be considered when treating patients with underlying cognitive or psychiatric disturbances.
Hepatic Insufficiency: Based on decreased plasma clearance observed in patients with moderate hepatic impairment, the starting dose in these patients should be 5 mg twice daily, followed by adjustment based on therapeutic response and tolerability. Patients with mild hepatic insufficiency (Child-Pugh score of 5-6) do not require dosage reductions. To date, pharmacokinetic studies in subjects with severe hepatic impairment (Child-Pugh score of 10-15) have not been carried out. The use of pilocarpine in these patients is not recommended.
Child-Pugh Scoring System for Hepatic ImpairmentClinical and Biochemical Measurements Points Scored for Increasing Abnormality
1
2
3Encephalopathy (grade)*
None
1 and 2
3 and 4Ascites
Absent
Slight
ModerateBilirubin (mg. per 100 mL)
1-2
2-3
>3Albumin (g. per 100 mL)
3-5
2.8-3.5
<2.8Prothrombin Time (sec. Prolonged)
1-4
4-6
>6For Primary Biliary Cirrhosis:-
Bilirubin (mg. per 100 mL)
1-4
4-10
>10* According to grading of Trey C, Burns D, and Saunders S. Treatment of hepatic coma by exchange blood transfusion. N Engl J Med. 1966; 274:473-481.
Reference: Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Brit J Surg. 1973; 60:646-9Information for Patients
Patients should be informed that pilocarpine may cause visual disturbances, especially at night, that could impair their ability to drive safely. If a patient sweats excessively while taking pilocarpine hydrochloride and cannot drink enough liquid, the patient should consult a physician. Dehydration may develop.
Drug Interactions
Pilocarpine should be administered with caution to patients taking beta-adrenergic antagonists because of the possibility of conduction disturbances. Drugs with parasympathomimetic effects administered concurrently with pilocarpine would be expected to result in additive pharmacologic effects. Pilocarpine might antagonize the anticholinergic effects of drugs used concomitantly. These effects should be considered when anticholinergic properties may be contributing to the therapeutic effect of concomitant medication (e.g., atropine, inhaled ipratropium). While no formal drug interaction studies have been performed, the following concomitant drugs were used in at least 10% of patients in either or both Sjogren's efficacy studies: acetylsalicylic acid, artificial tears, calcium, conjugated estrogens, hydroxychloroquine sulfate, ibuprofen, levothyroxine sodium, medroxyprogesterone acetate, methotrexate, multivitamins, naproxen, omeprazole, paracetamol, and prednisone.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime oral carcinogenicity studies were conducted in CD-1 mice and Sprague-Dawley rats. Pilocarpine did not induce tumors in mice at any dosage studied (up to 30 mg/kg/day, which yielded a systemic exposure approximately 50 times larger than the maximum systemic exposure observed clinically). In rats, a dosage of 18 mg/kg/day, which yielded a systemic exposure approximately 100 times larger than the maximum systemic exposure observed clinically, resulted in a statistically significant increase in the incidence of benign pheochromocytomas in both males and females, and a statistically significant increase in the incidence of hepatocellular adenomas in female rats. The tumorigenicity observed in rats was observed only at a large multiple of the maximum labeled clinical dose, and may not be relevant to clinical use.
No evidence that pilocarpine has the potential to cause genetic toxicity was obtained in a series of studies that included: 1) bacterial assays (Salmonella and E. coli) for reverse gene mutations; 2) an in vitro chromosome aberration assay in a Chinese hamster ovary cell line; 3) an in vivo chromosome aberration assay (micronucleus test) in mice; and 4) a primary DNA damage assay (unscheduled DNA synthesis) in rat hepatocyte primary cultures.
Oral administration of pilocarpine to male and female rats at a dosage of 18 mg/kg/day, which yielded a systemic exposure approximately 100 times larger than the maximum systemic exposure observed clinically, resulted in impaired reproductive function, including reduced fertility, decreased sperm motility, and morphologic evidence of abnormal sperm. It is unclear whether the reduction in fertility was due to effects on male animals, female animals, or both males and females. In dogs, exposure to pilocarpine at a dosage of 3 mg/kg/day (approximately 3 times the maximum recommended human dose when compared on the basis of body surface area (mg/m2) estimates) for six months resulted in evidence of impaired spermatogenesis. The data obtained in these studies suggest that pilocarpine may impair the fertility of male and female humans. SALAGEN® Tablets should be administered to individuals who are attempting to conceive a child only if the potential benefit justifies potential impairment of fertility.Pregnancy: Teratogenic Effects
Pilocarpine was associated with a reduction in the mean fetal body weight and an increase in the incidence of skeletal variations when given to pregnant rats at a dosage of 90 mg/kg/day (approximately 26 times the maximum recommended dose for a 50 kg human when compared on the basis of body surface area (mg/m2) estimates). These effects may have been secondary to maternal toxicity. In another study, oral administration of pilocarpine to female rats during gestation and lactation at a dosage of 36 mg/kg/day (approximately 10 times the maximum recommended dose for a 50 kg human when compared on the basis of body surface area (mg/m2) estimates) resulted in an increased incidence of stillbirths; decreased neonatal survival and reduced mean body weight of pups were observed at dosages of 18 mg/kg/day (approximately 5 times the maximum recommended dose for a 50 kg human when compared on the basis of body surface area (mg/m2) estimates) and above. There are no adequate and well-controlled studies in pregnant women. SALAGEN® Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from SALAGEN® Tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Head & Neck Cancer Patients: In the placebo-controlled clinical trials (See Clinical Studies section) the mean age of patients was approximately 58 years (range 19 to 80). Of these patients, 97/369 (61/217 receiving pilocarpine) were over the age of 65 years. In the healthy volunteer studies, 15/150 subjects were over the age of 65 years. In both study populations, the adverse events reported by those over 65 years and those 65 years and younger were comparable. Of the 15 elderly volunteers (5 women, 10 men), the 5 women had higher Cmax's and AUC's than the men. (See Pharmacokinetics section.)
Sjogren's Syndrome Patients: In the placebo-controlled clinical trials (See Clinical Studies section), the mean age of patients was approximately 55 years (range 21 to 85). The adverse events reported by those over 65 years and those 65 years and younger were comparable except for notable trends for urinary frequency, diarrhea, and dizziness (See ADVERSE REACTIONS section). -
ADVERSE REACTIONS
Head & Neck Cancer Patients: In controlled studies, 217 patients received pilocarpine, of whom 68% were men and 32% were women. Race distribution was 91% Caucasian, 8% Black, and 1% of other origin. Mean age was approximately 58 years. The majority of patients were between 50 and 64 years (51%), 33% were 65 years and older and 16% were younger than 50 years of age. The most frequent adverse experiences associated with SALAGEN® Tablets were a consequence of the expected pharmacologic effects of pilocarpine.
Adverse Event
Pilocarpine HCl
Placebo
10 mg t.i.d. (30 mg/day)
5 mg t.i.d. (15 mg/day)
(t.i.d.)Sweating
N = 121/68%
N = 141/29%
N = 152/9%Nausea
15
6
4Rhinitis
14
5
7Diarrhea
7
4
5Chills
15
3
<1Flushing
13
8
3Urinary Frequency
12
9
7Dizziness
12
5
4Asthenia
12
6
3In addition, the following adverse events (≥3% incidence) were reported at dosages of 15-30 mg/day in the controlled clinical trials:
Adverse Event
Pilocarpine HCl
Placebo
5-10 mg t.i.d. (15-30 mg/day)
(t.i.d.)Headache
N = 212/11%
N = 152/8%Dyspepsia
7
5Lacrimation
6
8Edema
5
4Abdominal Pain
4
4Amblyopia
4
2Vomiting
4
1Pharyngitis
3
8Hypertension
3
1
The following events were reported with treated head and neck cancer patients at incidences of 1% to 2% at dosages of 7.5 to 30 mg/day: abnormal vision, conjunctivitis, dysphagia, epistaxis, myalgias, pruritus, rash, sinusitis, tachycardia, taste perversion, tremor, voice alteration.
The following events were reported rarely in treated head and neck cancer patients (<1%): Causal relation is unknown.
Body as a whole: body odor, hypothermia, mucous membrane abnormality
Cardiovascular: bradycardia, ECG abnormality, palpitations, syncope
Digestive: anorexia, increased appetite, esophagitis, gastrointestinal disorder, tongue disorder
Hematologic: leukopenia, lymphadenopathy
Nervous: anxiety, confusion, depression, abnormal dreams, hyperkinesia, hypesthesia, nervousness, parethesias, speech disorder, twitching
Respiratory: increased sputum, stridor, yawning
Skin: seborrhea
Special senses: deafness, eye pain, glaucoma
Urogenital: dysuria, metrorrhagia, urinary impairment
In long-term treatment were two patients with underlying cardiovascular disease of whom one experienced a myocardial infarct and another an episode of syncope. The association with drug is uncertain.Sjogren's Syndrome Patients: In controlled studies, 376 patients received pilocarpine, of whom 5% were men and 95% were women. Race distribution was 84% Caucasian, 9% Oriental, 3% Black, and 4% of other origin. Mean age was 55 years. The majority of patients were between 40 and 69 years (70%), 16% were 70 years and older and 14% were younger than 40 years of age. Of these patients, 161/629 (89/376 receiving pilocarpine) were over the age of 65 years. The adverse events reported by those over 65 years and those 65 years and younger were comparable except for notable trends for urinary frequency, diarrhea, and dizziness. The incidences of urinary frequency and diarrhea in the elderly were about double those of the non-elderly. The incidence of dizziness was about three times as high in the elderly as in the non-elderly. These adverse experiences were not considered to be serious. In the 2 placebo-controlled studies, the most common adverse events related to drug use were sweating, urinary frequency, chills, and vasodilatation (flushing). The most commonly reported reason for patient discontinuation of treatment was sweating. Expected pharmacologic effects of pilocarpine include the following adverse experiences associated with SALAGEN® Tablets:
Adverse Event
Pilocarpine HCl
Placebo
5 mg q.i.d. (20 mg/day)
(q.i.d.)Sweating
N = 255/40%
N = 253/7%Urinary Frequency
10
4Nausea
9
9Flushing
9
2Rhinitis
7
8Diarrhea
6
7Chills
4
2Increased Salivation
3
0Asthenia
2
2
In addition, the following adverse events (≥3% incidence) were reported at dosages of 20 mg/day in the controlled clinical trials:
Adverse Event
Pilocarpine HCl
Placebo
5 mg q.i.d. (20 mg/day)
(q.i.d.)Headache
N = 255/13%
N = 253/19%Flu Syndrome
9
9Dyspepsia
7
7Dizziness
6
7Pain
4
2Sinusitis
4
5Abdominal Pain
3
4Vomiting
3
1Pharyngitis
2
5Rash
2
3Infection
2
6
The following events were reported in Sjogren's patients at incidences of 1% to 2% at dosing of 20 mg/day: accidental injury, allergic reaction, back pain, blurred vision, constipation, increased cough, edema, epistaxis, face edema, fever, flatulence, glossitis, lab test abnormalities, including chemistry, hematology, and urinalysis, myalgia, palpitation, pruritus, somnolence, stomatitis, tachycardia, tinnitus, urinary incontinence, urinary tract infection, and vaginitis.
The following events were reported rarely in treated Sjogren's patients (<1%) at dosing of 10-30 mg/day: Causal relation is unknown.
Body as a whole: chest pain, cyst, death, moniliasis, neck pain, neck rigidity, photosensitivity reaction
Cardiovascular: angina pectoris, arrhythmia, ECG abnormality, hypotension, hypertension, intracranial hemorrhage, migraine, myocardial infarction
Digestive: anorexia, bilirubinemia, cholelithiasis, colitis, dry mouth, eructation, gastritis, gastroenteritis, gastrointestinal disorder, gingivitis, hepatitis, abnormal liver function tests, melena, nausea & vomiting, pancreatitis, parotid gland enlargement, salivary gland enlargement, sputum increased, taste loss, tongue disorder, tooth disorder
Hematologic: hematuria, lymphadenopathy, abnormal platelets, thrombocythemia, thrombocytopenia, thrombosis, abnormal WBC
Metabolic and Nutritional: peripheral edema, hypoglycemia
Musculoskeletal: arthralgia, arthritis, bone disorder, spontaneous bone fracture, pathological fracture, myasthenia, tendon disorder, tenosynovitis
Nervous: aphasia, confusion, depression, abnormal dreams, emotional lability, hyperkinesia, hypesthesia, insomnia, leg cramps, nervousness, parethesias, abnormal thinking, tremor
Respiratory: bronchitis, dyspnea, hiccup, laryngismus, laryngitis, pneumonia, viral infection, voice alteration
Skin: alopecia, contact dermatitis, dry skin, eczema, erythema nodosum, exfoliative dermatitis, herpes simplex, skin ulcer, vesiculobullous rash
Special Senses: cataract, conjunctivitis, dry eyes, ear disorder, ear pain, eye disorder, eye hemorrhage, glaucoma, lacrimation disorder, retinal disorder, taste perversion, abnormal vision
Urogenital: breast pain, dysuria, mastitis, menorrhagia, metrorrhagia, ovarian disorder, pyuria, salpingitis, urethral pain, urinary urgency, vaginal hemorrhage, vaginal moniliasis
The following adverse experiences have been reported rarely with ocular pilocarpine: A-V block, agitation, ciliary congestion, confusion, delusion, depression, dermatitis, middle ear disturbance, eyelid twitching, malignant glaucoma, iris cysts, macular hole, shock, and visual hallucination.
To report SUSPECTED ADVERSE REACTIONS, contact Concordia Pharmaceuticals at 1-877-370-1142 of FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
MANAGEMENT OF OVERDOSE
Fatal overdosage with pilocarpine has been reported in the scientific literature at doses presumed to be greater than 100 mg in two hospitalized patients. 100 mg of pilocarpine is considered potentially fatal. Overdosage should be treated with atropine titration (0.5 mg to 1.0 mg given subcutaneously or intravenously) and supportive measures to maintain respiration and circulation. Epinephrine (0.3 mg to 1.0 mg, subcutaneously or intramuscularly) may also be of value in the presence of severe cardiovascular depression or bronchoconstriction. It is not known if pilocarpine is dialyzable.
-
DOSAGE AND ADMINISTRATION
Regardless of the indication, the starting dose in patients with moderate hepatic impairment should be 5 mg twice daily, followed by adjustment based on therapeutic response and tolerability. Patients with mild hepatic insufficiency do not require dosage reductions. The use of pilocarpine in patients with severe hepatic insufficiency is not recommended. If needed, refer to the Hepatic Insufficiency subsection of the Precautions section of this label for definitions of mild, moderate and severe hepatic impairment.
Head & Neck Cancer Patients: The recommended initial dose of SALAGEN® Tablets is 5 mg taken three times a day. Dosage should be titrated according to therapeutic response and tolerance. The usual dosage range is up to 15-30 mg per day. (Not to exceed 10 mg per dose.) Although early improvement may be realized, at least 12 weeks of uninterrupted therapy with SALAGEN® Tablets may be necessary to assess whether a beneficial response will be achieved. The incidence of the most common adverse events increases with dose. The lowest dose that is tolerated and effective should be used for maintenance.
Sjogren's Syndrome Patients: The recommended dose of SALAGEN® Tablets is 5 mg taken four times a day. Efficacy was established by 6 weeks of use. -
HOW SUPPLIED
SALAGEN® Tablets, 5 mg, are white, film coated, debossed round tablets, coded SAL 5. Each tablet contains 5 mg pilocarpine hydrochloride. They are supplied as follows: NDC: 59212-705-10 bottles of 100
Store up to 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
SALAGEN® Tablets, 7.5 mg, are blue, film coated, debossed round tablets, coded SAL 7.5. Each tablet contains 7.5 mg pilocarpine hydrochloride. They are supplied as follows: NDC: 59212-775-10 bottles of 100
Store up to 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
Manufactured by:
Patheon Inc.,
Ontario, L5N 7K9
Manufactured for:
Concordia Pharmaceuticals
Distributed by:
Amdipharm Limited
17 Northwood House
Dublin 9, Ireland
©2019. All rights reserved. Sep 2019
SALAGEN® is a registered trademark under exclusive license to Amdipharm Limited.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SALAGEN
pilocarpine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59212-705 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PILOCARPINE HYDROCHLORIDE (UNII: 0WW6D218XJ) (PILOCARPINE - UNII:01MI4Q9DI3) PILOCARPINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code SAL;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59212-705-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020237 09/01/2019 SALAGEN
pilocarpine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59212-775 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PILOCARPINE HYDROCHLORIDE (UNII: 0WW6D218XJ) (PILOCARPINE - UNII:01MI4Q9DI3) PILOCARPINE HYDROCHLORIDE 7.5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color BLUE Score no score Shape ROUND Size 6mm Flavor Imprint Code SAL;7;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59212-775-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020237 09/01/2019 Labeler - Concordia Pharmaceuticals Inc. (860243190)
Trademark Results [Salagen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SALAGEN 74120609 1753114 Live/Registered |
EISAI INC. 1990-12-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

