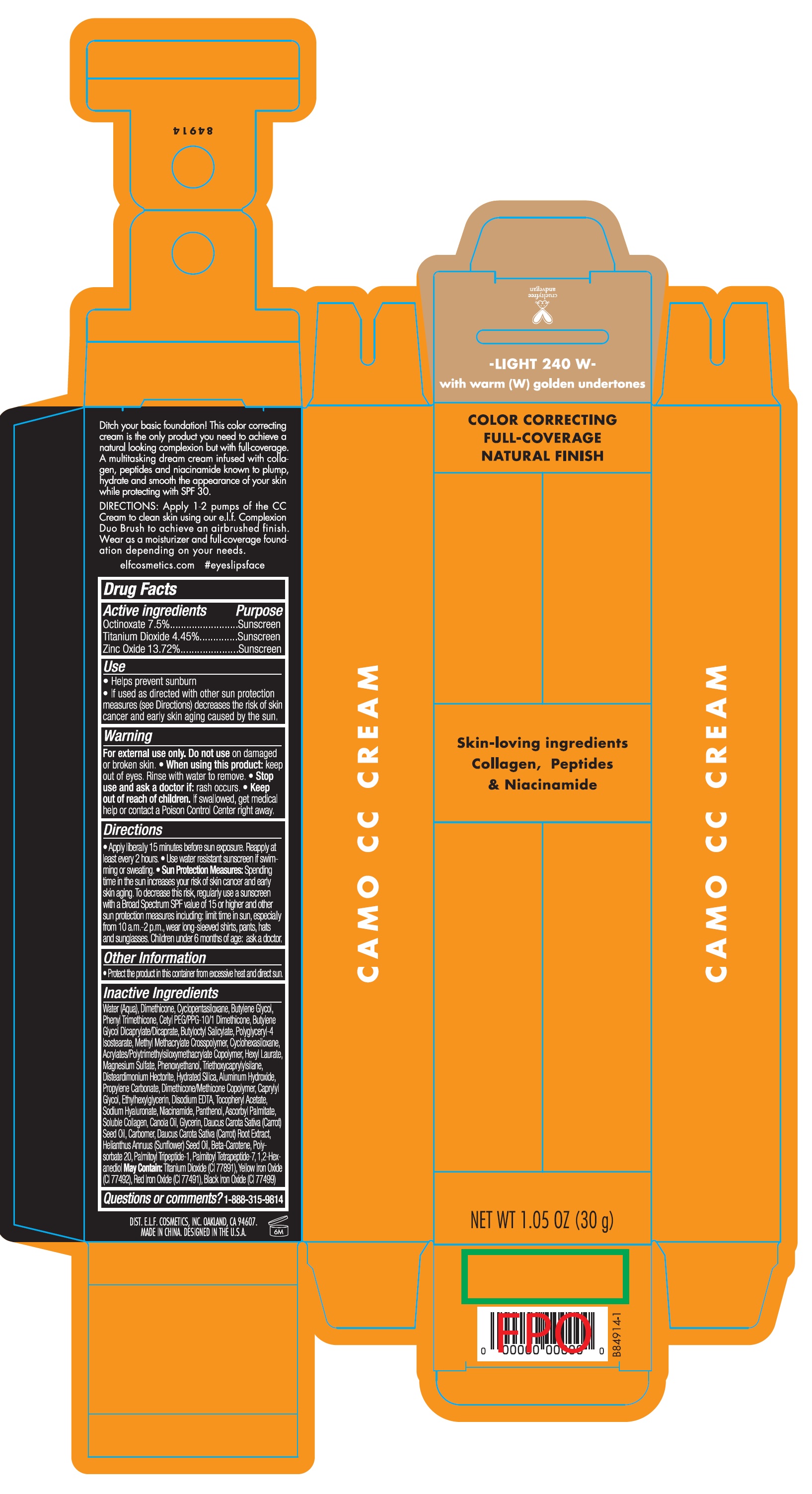
Camo CC Cream SPF30 Light 240 W
Camo CC Cream SPF30 Light 240 W by
Drug Labeling and Warnings
Camo CC Cream SPF30 Light 240 W by is a Otc medication manufactured, distributed, or labeled by e.l.f. Cosmetics, Inc, Zhejiang Ayan Biotech Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CAMO CC CREAM SPF30 LIGHT 240 W- octinoxate, titanium dioxide, zinc oxide cream
e.l.f. Cosmetics, Inc
----------
Camo CC Cream SPF30 Light 240 W
Use
- Helps prevent sunburn
- If used as directed with other sun protecion measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
- Use water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sun screen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in sun, especially from 10 a.m.-2 p.m. wear long- sleeved shirts, pants, hats and sunglasses. Children under 6 months of age: ask a doctor. Sun Protection Measures:
Inactive Ingredients
Water (Aqua), Dimethicone, Cylopentasiloxane, Butylene Glycol, Phenyl Trimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Butylene Glycol Dicaprylate/Dicaprate, Butyloctyl Salicylate, POlyglyceryl-4 Isostearate, Methyl Methacrylate Crosspolymer, Cyclohexasiloxane, Acrylates/Polytrimethylsiloxymethacrylate Copolymer, Hexyl Laurate, Magnesium Sulfate, Phenoxyethanol, Triethoxycaprylylylsilane, Disteardimonium Hectorite, Hydrated Silica, Aluminium Hydroxide, Propylene Carbonate, Dimethicone/Methicone Copolymer, Caprylyl Glycol, Ethylhexylglycerin, Disodium Edta, Tocopheryl Acetate, Sodium Hyaluronate, Niacinamide, Panthenol, Ascorbyl Palmitate, Soluble Collagen, Canola Oil, Glycerin, Daucu Carota Sativa (Carrot) See Oil, Carbomer, Daucus Carota Sativa (Carrot) Root Extract, Helianthus Annuus (Sunflower) Seed Oil, Beta-Carotene, Poly-sorbate 20, Palmitoyl Tripeptide-1, Palmitoyl Tetrapeptide-7, 1,2-Hexanediol Titanium Dioxide (CI 77891), Yellow Iron Oxide(CI 77492), Red Iron Oxide (CI77491), Black Iron Oxide (CI 77499)
May Contain:
| CAMO CC CREAM SPF30 LIGHT 240 W
octinoxate, titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - e.l.f. Cosmetics, Inc (093902816) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Ayan Biotech Co.,Ltd. | 544377996 | manufacture(76354-914) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.