ESTRUMATE- cloprostenol sodium injection
Estrumate by
Drug Labeling and Warnings
Estrumate by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
250 mcg cloprostenol/mL (equivalent to 263 mcg cloprostenol sodium/mL)
A sterile solution of a prostaglandin F2α analogue for intramuscular injection in beef cows, lactating dairy cows, and replacement beef and dairy heifers
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
-
DESCRIPTION
DESCRIPTION:
Estrumate® (cloprostenol injection) is a synthetic prostaglandin analogue structurally related to prostaglandin F2 α (PGF2 α). Each mL of the sterile colorless aqueous solution contains 250 mcg cloprostenol (equivalent to 263 mcg cloprostenol sodium), 6.1 mg sodium citrate, 0.56 mg anhydrous citric acid, 6.7 mg sodium chloride, 20 mg benzyl alcohol, and water for injection, q.s.
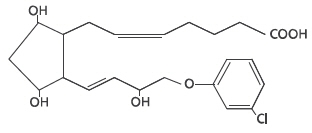
-
VETERINARY INDICATIONS
INDICATIONS FOR USE:
- For unobserved or non-detected estrus in beef cows, lactating dairy cows, and replacement beef and dairy heifers
- For treatment of pyometra or chronic endometritis in beef cows, lactating dairy cows, and replacement beef and dairy heifers
- For treatment of mummified fetus in beef cows, lactating dairy cows, and replacement beef and dairy heifers
- For treatment of luteal cysts in beef cows, lactating dairy cows, and replacement beef and dairy heifers
- For abortion of beef cows, lactating dairy cows, and replacement beef and dairy heifers
- For estrus synchronization in beef cows, lactating dairy cows, and replacement beef and dairy heifers
- For use with Fertagyl® (gonadorelin) to synchronize estrous cycles to allow for fixed time artificial insemination (FTAI) in lactating dairy cows.
Estrumate causes functional and morphological regression of the corpus luteum (luteolysis) in cattle. In normal, non-pregnant cycling animals, this effect on the life span of the corpus luteum usually results in estrus 2 to 5 days after treatment. In animals with prolonged luteal function (pyometra, mummified fetus, and luteal cysts), the induced luteolysis usually results in resolution of the condition and return to cyclicity. Pregnant animals may abort depending on the stage of gestation.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
Two mL of Estrumate (500 mcg cloprostenol) should be administered by INTRAMUSCULAR INJECTION using the specific dosage regimen for the indication.
20 mL bottle size: Use within 28 days of first puncture.
100 mL bottle size: Use within 28 days of first puncture and puncture a maximum of 12 times. Use only with automatic injection equipment or repeater syringe. Discard bottle after one stopper puncture with draw-off spike.
-
For unobserved or non-detected estrus in beef cows, lactating dairy cows, and replacement beef and dairy heifers
Cows and heifers which are not detected in estrus, although ovarian cyclicity continues, can be treated with Estrumate if a mature corpus luteum is present. Estrus is expected to occur 2 to 5 days following injection, at which time animals may be inseminated. Treated cattle should be inseminated at the usual time following detection of estrus. If estrous detection is not desirable or possible, treated animals may be inseminated twice at about 72 and 96 hours post-injection. -
For treatment of pyometra or chronic endometritis in beef cows, lactating dairy cows, and replacement beef and dairy heifers
Damage to the reproductive tract at calving or postpartum retention of the placenta often leads to infection and inflammation of the uterus (endometritis). Under certain circumstances, this may progress into chronic endometritis with the uterus becoming distended with purulent matter. This condition, commonly referred to as pyometra, is characterized by a lack of cyclical estrous behavior and the presence of a persistent corpus luteum. Induction of luteolysis with Estrumate usually results in evacuation of the uterus and a return to normal cyclical activity within 14 days after treatment. After 14 days post-treatment, recovery rate of treated animals will not be different than that of untreated cattle. -
For treatment of mummified fetus in beef cows, lactating dairy cows, and replacement beef and dairy heifers
Death of the conceptus during gestation may be followed by its degeneration and dehydration. Induction of luteolysis with Estrumate usually results in expulsion of the mummified fetus from the uterus. (Manual assistance may be necessary to remove the fetus from the vagina). Normal cyclical activity usually follows. -
For treatment of luteal cysts in beef cows, lactating dairy cows, and replacement beef and dairy heifers
A cow or heifer may be noncyclic due to the presence of a luteal cyst (a single, anovulatory follicle with a thickened wall which is accompanied by no external signs and by no changes in palpable consistency of the uterus). Treatment with Estrumate can restore normal ovarian activity by causing regression of the luteal cyst. -
For abortion of beef cows, lactating dairy cows, and replacement beef and dairy heifers
Unwanted pregnancies can be safely and efficiently terminated from 1 week after mating until about 5 months of gestation. The induced abortion is normally uncomplicated and the fetus and placenta are usually expelled about 4 to 5 days after the injection with the reproductive tract returning to normal soon after the abortion. The ability of Estrumate to induce abortion decreases beyond the fifth month of gestation while the risk of dystocia and its consequences increases. Estrumate has not been sufficiently tested under feedlot conditions; therefore, recommendations cannot be made for its use in heifers placed in feedlots. -
For estrus synchronization in beef cows, lactating dairy cows, and replacement beef and dairy heifers
The luteolytic action of Estrumate can be utilized to schedule estrus and ovulation for an individual cycling animal or a group of animals. This allows control of the time at which cycling cows or heifers can be bred. Estrumate can be used in a breeding program with the following methods:- Single Estrumate injection: Only animals with a mature corpus luteum should be treated to obtain maximum response to the single injection. However, not all cycling cattle should be treated since a mature corpus luteum is present for only 11 to 12 days of the 21-day cycle. Prior to treatment, cattle should be examined rectally and found to be anatomically normal, be non-pregnant, and have a mature corpus luteum. If these criteria are met, estrus is expected to occur 2 to 5 days following injection, at which time animals may be inseminated. Treated cattle should be inseminated at the usual time following detection of estrus. If estrous detection is not desirable or possible, treated animals may be inseminated either once at about 72 hours or twice at about 72 and 96 hours post-injection. With a single injection program, it may be desirable to assess the cyclicity status of the herd before Estrumate treatment. This can be accomplished by heat detecting and breeding at the usual time following detection of estrus for a 6-day period, all prior to injection. If by the sixth day the cyclicity status appears normal (approximately 25%-30% detected in estrus), all cattle not already inseminated should be palpated for normality, non-pregnancy, and cyclicity, then injected with Estrumate. Breeding should then be continued at the usual time following signs of estrus on the seventh and eighth days. On the ninth and tenth days, breeding may continue at the usual time following detection of estrus, or all cattle not already inseminated may be bred either once on the ninth day (at about 72 hours post-injection) or on both the ninth and tenth days (at about 72 and 96 hours post-injection).
- Double Estrumate injections: prior to treatment, cattle should be examined rectally and found to be anatomically normal, nonpregnant, and cycling (the presence of a mature corpus luteum is not necessary when the first injection of a double injection regimen is given). A second injection should be given 11 days after the first injection. In normal, cycling cattle, estrus is expected 2 to 5 days following the second injection. Treated cattle should be inseminated at the usual time following detection of estrus. If estrous detection is not desirable or possible, treated animals may be inseminated either once at about 72 hours or twice at about 72 and 96 hours following the second Estrumate injection. Many animals will come into estrus following the first injection; these animals can be inseminated at the usual time following detected estrus. Animals not inseminated should receive a second injection 11 days after the first injection. Animals receiving both injections may be inseminated at the usual time following detection of estrus or may be inseminated either once at about 72 hours or twice at about 72 and 96 hours post second injection.
Any breeding program recommended should be completed by either:
- observing animals (especially during the third week after injection) and inseminating or hand mating any animals returning to estrus, or
- turning in clean-up bull(s) 5 to 7 days after the last injection of Estrumate to cover any animals returning to estrus.
Management considerations for use of Estrumate for estrus synchronization:
A variety of programs can be designed to best meet the needs of individual management systems. A breeding program should be selected which is appropriate for the existing circumstances and management practices. Before a breeding program is planned, the producer's objectives must be examined and the producer must be made aware of the projected results and limitations. The producer and the consulting veterinarian should review the operation's breeding history, herd health, and nutritional status and agree that a breeding program is practical in the producer's specific situation. For any successful breeding program:- cows and heifers must be normal, non-pregnant, and cycling (rectal palpation should be performed);
- cows and heifers must be in sound breeding condition and on an adequate or increasing plane of nutrition;
- proper program planning and record keeping are essential;
- if artificial insemination is used, it must be performed by competent inseminators using high-quality semen.
It is important to understand that Estrumate is effective only in animals with a mature corpus luteum (ovulation must have occurred at least 5 days prior to treatment). This must be considered when breeding is intended following a single Estrumate injection.
There is no difference in the fertility achieved following the single or double dosage regimen when breeding occurs at induced estrus, or at 72 and 96 hours post-treatment. Conception rates may be lower than expected in those fixed time breeding programs employing Estrumate alone which omit the second insemination (ie, the insemination at or near 96 hours). This is especially true if a fixed time insemination is used following a single Estrumate injection.
-
For use with Fertagyl® (gonadorelin) to synchronize estrous cycles to allow for fixed time artificial insemination (FTAI) in lactating dairy cows
Use in reproductive synchrony programs similar to the following:- Administer the first Fertagyl® injection (2 mL; 86 mcg gonadorelin, as gonadorelin acetate) by intramuscular injection on Day 0.
- Administer 2 mL of Estrumate by intramuscular injection 6 to 8 days after the first Fertagyl® injection.
- Administer the second Fertagyl® injection (2mL; 86 mcg gonadorelin, as gonadorelin acetate) 30 to 72 hours after the Estrumate injection.
- Perform FTAI 8 to 24 hours after the second Fertagyl® injection, or inseminate cows on detected estrus using standard herd practices.
-
For unobserved or non-detected estrus in beef cows, lactating dairy cows, and replacement beef and dairy heifers
- CONTRAINDICATIONS
- WARNINGS
-
SAFE HANDLING WARNING
USER SAFETY WARNINGS: Not for use in humans. Keep this and all drugs out of the reach of children.
Women of childbearing age, asthmatics, and persons with bronchial and other respiratory problems should exercise extreme caution when handling this product.
Estrumate is readily absorbed through the skin and can cause abortion and/or bronchospasms. Direct contact with the skin should therefore be avoided. Accidental spillage on the skin should be washed off immediately with soap and water.
To obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, contact Merck Animal Health at 1-800-211-3573 or http://www.merck.com
-
SPL UNCLASSIFIED SECTION
ANIMAL SAFETY WARNINGS:
As with all parenteral products, careful aseptic techniques should be employed to decrease the possibility of post-injection bacterial infection. Severe localized clostridial infections associated with injection of Estrumate have been reported. In rare instances, such infections have resulted in death. Aggressive antibiotic therapy should be employed at the first sign of infection at the injection site, whether localized or diffuse.
At 50 and 100 times the recommended dose, mild side effects may be detected in some cattle. These include increased uneasiness, slight frothing, and milk let-down.
- SPL UNCLASSIFIED SECTION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 20 mL Vial Carton
NDC: 0061-5995-01
10 Doses
20 mLEstrumate®
(cloprostenol injection)250 mcg cloprostenol/mL
(equivalent to 263 mcg
cloprostenol sodium/mL)A sterile solution of a
prostaglandin F2α analogue
for intramuscular injection
in beef cows, lactating dairy
cows, and replacement beef
and dairy heifers.CAUTION: Federal (USA) law
restricts this drug to use by or
on the order of a licensed
veterinarian.MERCK
Animal Health
-
INGREDIENTS AND APPEARANCE
ESTRUMATE
cloprostenol sodium injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5995 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength cloprostenol sodium (UNII: 886SAV9675) (cloprostenol - UNII:4208238832) cloprostenol 263 ug in 1 mL Inactive Ingredients Ingredient Name Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) Anhydrous Citric Acid (UNII: XF417D3PSL) Sodium Chloride (UNII: 451W47IQ8X) Benzyl alcohol (UNII: LKG8494WBH) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5995-01 1 in 1 CARTON 1 20 mL in 1 VIAL, MULTI-DOSE 2 NDC: 0061-5995-02 1 in 1 CARTON 2 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA113645 02/02/1982 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Vet Pharma Friesoythe GmbH 341934053 MANUFACTURE Establishment Name Address ID/FEI Business Operations Piramal Healthcare Uklimited 215586998 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Cayman Pharma s.r.o. 495076551 API MANUFACTURE
Trademark Results [Estrumate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ESTRUMATE 77084378 not registered Dead/Abandoned |
INTERVET INC. 2007-01-17 |
 ESTRUMATE 73086007 1102497 Live/Registered |
IMPERIAL CHEMICAL INDUSTRIES LIMITED 1976-05-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.