Benefit BOO BOO ZAP Medicated Acne Treatment
Benefit BOO BOO ZAP Medicated Acne Treatment by
Drug Labeling and Warnings
Benefit BOO BOO ZAP Medicated Acne Treatment by is a Otc medication manufactured, distributed, or labeled by Benefit Cosmetics, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BENEFIT BOO BOO ZAP MEDICATED ACNE TREATMENT- salicylic acid gel
Benefit Cosmetics, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
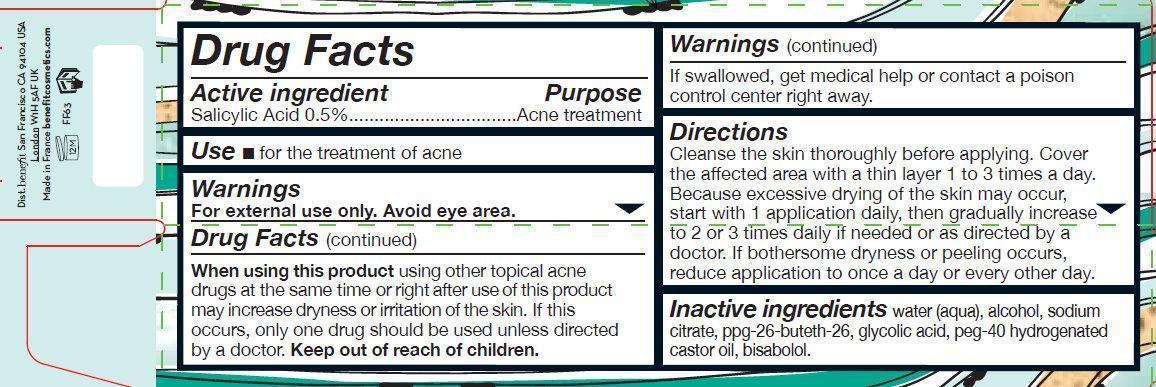
Benefit BOO BOO ZAP Medicated Acne Treatment
Warnings
For external use only. Avoid eye area.
Directions
Cleanse the skin thoroughly before applying. Cover the affected area with a thin layer 1 to 3 times a day. Because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
water (aqua), alcohol, sodium citrate, ppg-26-buteth-26, glycolic acid, peg-40 hydrogenated castor oil, bisabolol.
| BENEFIT BOO BOO ZAP MEDICATED ACNE TREATMENT
salicylic acid gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Benefit Cosmetics, LLC (070826813) |
| Registrant - Benefit Cosmetics, LLC (070826813) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.