FINACEA- azelaic acid gel FINACEA- azelaic acid kit
Finacea by
Drug Labeling and Warnings
Finacea by is a Prescription medication manufactured, distributed, or labeled by Intendis Inc., Intendis Manufacturing SPA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
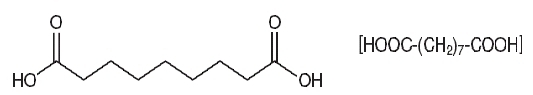
FINACEA® (azelaic acid) Gel, 15%, contains azelaic acid, a naturally occurring saturated dicarboxylic acid. Chemically, azelaic acid is 1,7-heptanedicarboxylic acid, with the molecular formula C9 H16 O4, a molecular weight of 188.22, and the structural formula:
Azelaic acid is a white, odorless crystalline solid that is poorly soluble in water at 20°C (0.24%), but freely soluble in boiling water and in ethanol.
Each gram of FINACEA Gel, 15%, contains 0.15 gm azelaic acid (15% w/w) as the active ingredient in an aqueous gel base containing benzoic acid (as a preservative), disodium EDTA, lecithin, medium-chain triglycerides, polyacrylic acid, polysorbate 80, propylene glycol, purified water, and sodium hydroxide to adjust pH.
-
CLINICAL PHARMACOLOGY
The mechanism(s) by which azelaic acid interferes with the pathogenic events in rosacea are unknown.
Pharmacokinetics:
The percutaneous absorption of azelaic acid after topical application of FINACEA Gel, 15%, could not be reliably determined. Mean plasma azelaic acid concentrations in rosacea patients treated with FINACEA Gel, 15%, twice daily for at least 8 weeks are in the range of 42 to 63.1 ng/mL. These values are within the maximum concentration range of 24.0 to 90.5 ng/mL observed in rosacea patients treated with vehicle only. This indicates that FINACEA Gel, 15%, does not increase plasma azelaic acid concentration beyond the range derived from nutrition and endogenous metabolism.
In vitro and human data suggest negligible cutaneous metabolism of 3H-azelaic acid 20% cream after topical application. Azelaic acid is mainly excreted unchanged in the urine, but undergoes some ß-oxidation to shorter chain dicarboxylic acids.
-
CLINICAL STUDIES
FINACEA Gel, 15%, was evaluated for the treatment of mild to moderate papulopustular rosacea in 2 clinical trials comprising a total of 664 (333 active to 331 vehicle) patients. Both trials were multicenter, randomized, double-blind, vehicle-controlled 12-week studies with identical protocols. Overall, 92.5% of patients were Caucasian and 73% of patients were women, and the mean age was 49 (range 21 to 86) years. Enrolled patients had mild to moderate rosacea with a mean lesion count of 18 (range 8 to 60) inflammatory papules and pustules. Subjects without papules and pustules, with nodules, rhinophyma, or ocular involvement, and a history of hypersensitivity to propylene glycol or to any other ingredients of the study drug were excluded. FINACEA Gel, 15%, or its vehicle were to be applied twice daily for 12 weeks; no other topical or systemic medication affecting the course of rosacea and/or evaluability was to be used during the studies. Patients were instructed to avoid spicy foods, thermally hot foods and drinks, and alcoholic beverages during the study, and to use only very mild soaps or soapless cleansing lotion for facial cleansing.
The primary efficacy endpoints were both
- 1. change from baseline in inflammatory lesion counts and
- 2. success defined as a score of clear or minimal with at least a 2 step reduction from baseline on the Investigator's Global Assessment (IGA):
CLEAR:
No papules and/or pustules; no or residual erythema; no or mild to moderate telangiectasia
MINIMAL:
Rare papules and/or pustules; residual to mild erythema; mild to moderate telangiectasia
MILD:
Few papules and/or pustules; mild erythema; mild to moderate telangiectasia
MILD TO MODERATE:
Distinct number of papules and/or pustules; mild to moderate erythema; mild to moderate telangiectasia
MODERATE:
Pronounced number of papules and/or pustules; moderate erythema; mild to moderate telangiectasia
MODERATE TO SEVERE:
Many papules and/or pustules, occasionally with large inflamed lesions; moderate erythema; moderate degree of telangiectasia
SEVERE:
Numerous papules and/or pustules, occasionally with confluent areas of inflamed lesions; moderate or severe erythema; moderate or severe telangiectasia
Primary efficacy assessment was based on the intent-to-treat (ITT) population with last observation carried forward (LOCF).
Both studies demonstrated a statistically significant difference in favor of FINACEA Gel, 15%, over its vehicle in reducing the number of inflammatory papules and pustules associated with rosacea (Table 1) and with success on the IGA in the ITT-LOCF population at the end of treatment.
Table 1. Inflammatory Papules and Pustules (ITT population)* - * ITT population with last observation carried forward (LOCF);
Study One
FINACEA Gel,15%
N = 164
Study One
VEHICLE
N = 165
Study Two
FINACEA Gel,15%
N = 167
Study Two
VEHICLE
N = 166
Mean Lesion
Count
Baseline
17.5
17.6
17.9
18.5
End of
Treatment*
6.8
10.5
9.0
12.1
Mean Percent
Reduction
End of
Treatment*
57.9%
39.9%
50.0%
38.2%
Although some reduction of erythema which was present in patients with papules and pustules of rosacea occurred in clinical studies, efficacy for treatment of erythema in rosacea in the absence of papules and pustules has not been evaluated.
FINACEA Gel, 15%, was superior to the vehicle with regard to success based on the investigator's global assessment of rosacea on a 7-point static score at the end of treatment, (ITT population; Table 2).
Table 2. Investigator's Global Assessment at the End of Treatment* - * ITT population with last observation carried forward (LOCF);
Study One
FINACEA Gel, 15%
N = 164
Study One
VEHICLE
N = 165
Study Two
FINACEA Gel, 15%
N = 167
Study Two
VEHICLE
N = 166
CLEAR, MINIMAL
or MILD at End
of Treatment
(%of Patients)
61%
40%
61%
48%
-
INDICATIONS AND USAGE
FINACEA Gel, 15%, is indicated for topical treatment of inflammatory papules and pustules of mild to moderate rosacea. Although some reduction of erythema which was present in patients with papules and pustules of rosacea occurred in clinical studies, efficacy for treatment of erythema in rosacea in the absence of papules and pustules has not been evaluated. Patients should be instructed to avoid spicy foods, thermally hot foods and drinks, alcoholic beverages and to use only very mild soaps or soapless cleansing lotion for facial cleansing.
- CONTRAINDICATIONS
-
WARNINGS
FINACEA Gel, 15%, is for dermatologic use only, and not for ophthalmic, oral or intravaginal use.
There have been isolated reports of hypopigmentation after use of azelaic acid. Since azelaic acid has not been well studied in patients with dark complexion, these patients should be monitored for early signs of hypopigmentation.
-
PRECAUTIONS
General:
Contact with the eyes should be avoided. If sensitivity or severe irritation develops with the use of FINACEA Gel, 15%, treatment should be discontinued and appropriate therapy instituted.
In a transgenic mouse study, chronic use of FINACEA Gel led to an increased number of animals with papillomas at the treatment site (see PRECAUTIONS: Carcinogenesis, Mutagenesis, and Impairment of Fertility). The clinical relevance of the findings in animal studies to humans is not clear.
Information for Patients
Patients using FINACEA Gel, 15%, should receive the following information and instructions:
- FINACEA Gel, 15%, is to be used only as directed by the physician.
- FINACEA Gel, 15%, is for external use only. It is not to be used orally, intravaginally, or for the eyes.
- Cleanse affected area(s) with a very mild soap or a soapless cleansing lotion and pat dry with a soft towel before applying FINACEA Gel, 15%. Avoid alcoholic cleansers, tinctures and astringents, abrasives and peeling agents.
- Avoid contact of FINACEA Gel, 15%, with the mouth, eyes and other mucous membranes. If it does come in contact with the eyes, wash the eyes with large amounts of water and consult a physician if eye irritation persists.
- The hands should be washed following application of FINACEA Gel, 15%.
- Cosmetics may be applied after FINACEA Gel, 15%, has dried.
- Skin irritation (e.g., pruritus, burning, or stinging) may occur during use of FINACEA Gel, 15%, usually during the first few weeks of treatment. If irritation is excessive or persists, use of FINACEA Gel, 15%, should be discontinued, and patients should consult their physician (see ADVERSE REACTIONS).
- Avoid any foods and beverages that might provoke erythema, flushing, and blushing (including spicy food, alcoholic beverages, and thermally hot drinks, including hot coffee and tea).
- Patients should report abnormal changes in skin color to their physician.
- Avoid the use of occlusive dressings or wrappings.
Drug Interactions:
There have been no formal studies of the interaction of FINACEA Gel, 15%, with other drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Systemic long-term animal studies have not been performed to evaluate the carcinogenic potential of azelaic acid. In a 26-week dermal carcinogenicity study using transgenic (Tg.AC) mice, FINACEA Gel, 15%, and the gel vehicle, when applied once or twice daily, did not increase the number of female Tg.AC animals with papillomas at the treatment site. No statistically significant increase in the number of animals with papillomas at the treatment site was observed in male Tg.AC animals after once daily application. After twice daily application, FINACEA Gel, 15%, and the gel vehicle induced a statistically significant increase in the number of male animals with papillomas at the treatment site when compared to untreated males. This suggests that the positive effect may be associated with the vehicle application. The clinical relevance of the findings in animals to humans is not clear.
Azelaic acid was not mutagenic or clastogenic in a battery of in vitro (Ames assay, HGPRT in V79 cells {Chinese hamster lung cells}, and chromosomal aberration assay in human lymphocytes) and in vivo (dominant lethal assay in mice and mouse micronucleus assay) genotoxicity tests.
Oral administration of azelaic acid at dose levels up to 2500 mg/kg/day (162 times the maximum recommended human dose based on body surface area) did not affect fertility or reproductive performance in male or female rats.
Pregnancy:
Teratogenic Effects
Pregnancy Category B
There are no adequate and well-controlled studies of topically administered azelaic acid in pregnant women. The experience with FINACEA Gel, 15%, when used by pregnant women is too limited to permit assessment of the safety of its use during pregnancy.
Dermal embryofetal developmental toxicology studies have not been performed with azelaic acid, 15%, gel. Oral embryofetal developmental studies were conducted with azelaic acid in rats, rabbits, and cynomolgus monkeys. Azelaic acid was administered during the period of organogenesis in all three animal species. Embryotoxicity was observed in rats, rabbits, and monkeys at oral doses of azelaic acid that generated some maternal toxicity. Embryotoxicity was observed in rats given 2500 mg/kg/day (162 times the maximum recommended human dose based on body surface area), rabbits given 150 or 500 mg/kg/day (19 or 65 times the maximum recommended human dose based on body surface area) and cynomolgus monkeys given 500 mg/kg/day (65 times the maximum recommended human dose based on body surface area) azelaic acid. No teratogenic effects were observed in the oral embryofetal developmental studies conducted in rats, rabbits and cynomolgus monkeys.
An oral peri- and post-natal developmental study was conducted in rats. Azelaic acid was administered from gestational day 15 through day 21 postpartum up to a dose level of 2500 mg/kg/day. Embryotoxicity was observed in rats at an oral dose that generated some maternal toxicity (2500 mg/kg/day; 162 times the maximum recommended human dose based on body surface area). In addition, slight disturbances in the postnatal development of fetuses was noted in rats at oral doses that generated some maternal toxicity (500 and 2500 mg/kg/day; 32 and 162 times the maximum recommended human dose based on body surface area). No effects on sexual maturation of the fetuses were noted in this study.
Because animal reproduction studies are not always predictive of human response, this drug should be used only if clearly needed during pregnancy.
Nursing Mothers:
Equilibrium dialysis was used to assess human milk partitioning in vitro. At an azelaic acid concentration of 25 μg/mL, the milk/ plasma distribution coefficient was 0.7 and the milk/buffer distribution was 1.0, indicating that passage of drug into maternal milk may occur. Since less than 4% of a topically applied dose of azelaic acid cream, 20%, is systemically absorbed, the uptake of azelaic acid into maternal milk is not expected to cause a significant change from baseline azelaic acid levels in the milk. However, caution should be exercised when FINACEA Gel, 15%, is administered to a nursing mother.
-
ADVERSE REACTIONS
Overall, treatment related adverse events, including burning, stinging/tingling, dryness/tightness/scaling, itching, and erythema/irritation/redness, were 19.4% (24/124) for FINACEA Gel, 15%, and 7.1% (9/127) for the active comparator gel at 15 weeks.
In two vehicle controlled, and one active controlled U.S. clinical studies, treatment safety was monitored in 788 patients who used twice daily FINACEA Gel, 15%, for 12 weeks (N=333) or for 15 weeks (N=124), or the gel vehicle (N=331) for 12 weeks.
Table 3. Cutaneous Adverse Events Occurring in ≥1% of Subjects in the Rosacea Trials by Treatment Group and Maximum Intensity* - * Subjects may have >1 cutaneous adverse event; thus, the sum of the frequencies of preferred terms may exceed the number of subjects with at least 1 cutaneous adverse event.
FINACEA Gel, 15%
N=457 (100%)
Vehicle
N=331 (100%)
Mild
n=99
(22%)
Moderate
n=61
(13%)
Severe
n=27
(6%)
Mild
n=46
(14%)
Moderate
n=30
(9%)
Severe
n=5
(2%)
Burning/
stinging/
tingling
71 (16%)
42 (9%)
17 (4%)
8 (2%)
6 (2%)
2 (1%)
Pruritus
29 (6%)
18 (4%)
5 (1%)
9 (3%)
6 (2%)
0 (0%)
Scaling/dry
skin/xerosis
21 (5%)
10 (2%)
5 (1%)
31 (9%)
14 (4%)
1 (<1%)
Erythema/
irritation
6 (1%)
7 (2%)
2 (<1%)
8 (2%)
4 (1%)
2 (1%)
Contact
dermatitis
2 (<1%)
3 (1%)
0 (0%)
1 (<1%)
0 (0%)
0 (0%)
Edema
3 (1%)
2 (<1%)
0 (0%)
3 (1%)
0 (0%)
0 (0%)
Acne
3 (1%)
1 (<1%)
0 (0%)
1 (<1%)
0 (0%)
0 (0%)
FINACEA Gel, 15%, and its vehicle caused irritant reactions at the application site in human dermal safety studies. FINACEA Gel, 15%, caused significantly more irritation than its vehicle in a cumulative irritation study. Some improvement in irritation was demonstrated over the course of the clinical studies, but this improvement might be attributed to subject dropouts. No phototoxicity or photoallergenicity were reported in human dermal safety studies.
In patients using azelaic acid formulations, the following additional adverse experiences have been reported rarely: worsening of asthma, vitiligo depigmentation, small depigmented spots, hypertrichosis, reddening (signs of keratosis pilaris), and exacerbation of recurrent herpes labialis.
Post-marketing safety-Skin: facial burning and irritation; Eyes: iridocyclitis on accidental exposure with FINACEA Gel, 15%, to the eye (see PRECAUTIONS).
-
OVERDOSAGE
FINACEA Gel, 15%, is intended for cutaneous use only. If pronounced local irritation occurs, patients should be directed to discontinue use and appropriate therapy should be instituted (See PRECAUTIONS).
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
FINACEA Gel, 15%, is supplied in tubes in the following size:
FINACEA Gel, 15% – 50 g tube – NDC: 10922-825-02
Storage
Store at 25°C (77°F); excursions permitted between 15–30°C (59–86°F) [see USP Controlled Room Temperature].
Distributed under license; U.S. Patent No 6,534,070
www.myfinacea.com
© 2010, Intendis, Inc. All rights reserved. July 2010
Manufactured by Intendis Manufacturing S.p.A., Segrate, Milan, Italy
Distributed by:
Intendis
Morristown, NJ 07962Intendis is part of the Bayer Group
6706803
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Finacea plus
NDC: 10922-826-10
Rx only
Finacea (azelaic acid) Gel, 15%–PlusDispense as a Complete Package
Package contains: Finacea® (azelaic acid) Gel, 15%, 50 g
For Dermatologic Use Only - Not For Ophthalmic Use
Complimentary CeraVe® Hydrating Cleanser 3 fl oz (87 mL)
CeraVe® Moisturizing Lotion 3 fl oz (87 mL)
Patient Information Brochurewww.myfinacea.com
-
PRINCIPAL DISPLAY PANEL
Finacea Gel, 15%–50 g Tube
NDC: 10922-825-02
For Dermatologic Use Only
Not For Ophthalmic UseFinacea®
(azelaic acid) Gel 15%50 grams
-
INGREDIENTS AND APPEARANCE
FINACEA
azelaic acid gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10922-825 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZELAIC ACID (UNII: F2VW3D43YT) (AZELAIC ACID - UNII:F2VW3D43YT) AZELAIC ACID 0.15 g in 1 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) EDETATE DISODIUM (UNII: 7FLD91C86K) 1,2-DIARACHIDOYL-SN-GLYCERO-3-PHOSPHOCHOLINE (UNII: HE0P2D9ZLS) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10922-825-02 1 in 1 CARTON 1 50 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021470 12/24/2004 FINACEA
azelaic acid kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10922-826 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10922-826-10 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 50 g Part 2 1 CONTAINER 87 mL Part 3 1 CONTAINER 87 mL Part 1 of 3 FINACEA
azelaic acid gelProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZELAIC ACID (UNII: F2VW3D43YT) (AZELAIC ACID - UNII:F2VW3D43YT) AZELAIC ACID 0.15 g in 1 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) EDETATE DISODIUM (UNII: 7FLD91C86K) 1,2-DIARACHIDOYL-SN-GLYCERO-3-PHOSPHOCHOLINE (UNII: HE0P2D9ZLS) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 50 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021470 12/24/2004 Part 2 of 3 HYDRATING CLEANSER
hydrating cleanser liquidProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) HYALURONIC ACID (UNII: S270N0TRQY) CHOLESTEROL (UNII: 97C5T2UQ7J) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) CETYL ALCOHOL (UNII: 936JST6JCN) EDETATE DISODIUM (UNII: 7FLD91C86K) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 87 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 04/12/2011 Part 3 of 3 MOISTURIZIER
moisturizer lotionProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) HYALURONIC ACID (UNII: S270N0TRQY) CHOLESTEROL (UNII: 97C5T2UQ7J) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) CETYL ALCOHOL (UNII: 936JST6JCN) EDETATE DISODIUM (UNII: 7FLD91C86K) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 87 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 04/12/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021470 04/12/2011 Labeler - Intendis Inc. (363706040) Registrant - Intendis Inc. (363706040) Establishment Name Address ID/FEI Business Operations Intendis Manufacturing SPA 564725468 MANUFACTURE(10922-825, 10922-826)
Trademark Results [Finacea]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FINACEA 76325515 2811242 Live/Registered |
LEO PHARMA A/S 2001-10-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.