VIJOICE- alpelisib tablet VIJOICE- alpelisib kit VIJOICE- alpelisib granule
VIJOICE by
Drug Labeling and Warnings
VIJOICE by is a Prescription medication manufactured, distributed, or labeled by Novartis Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIJOICE safely and effectively. See full prescribing information for VIJOICE.
VIJOICE® (alpelisib) tablets, for oral use
VIJOICE® (alpelisib) oral granules
Initial U.S. Approval: 2019
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
VIJOICE is a kinase inhibitor indicated for the treatment of adult and pediatric patients 2 years of age and older with severe manifestations of PIK3CA-Related Overgrowth Spectrum (PROS) who require systemic therapy.
This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
DOSAGE AND ADMINISTRATION
Recommended Dose:
- Pediatric patients (2 to less than 18 years of age): 50 mg taken orally once daily with food. (2.1)
- Adult patients: 250 mg taken orally once daily with food. (2.1)
Administration:
- Swallow tablets whole. Do not chew, divide, or crush. (2.4)
- For patients who cannot swallow tablets whole, use tablets or oral granules to create a suspension or a mixture. (2.4)
- VIJOICE suspension made with water can be administered orally or via a nasogastric or gastric tube. (2.4)
See full prescribing information for preparation and administration instructions.
CONTRAINDICATIONS
Severe hypersensitivity to VIJOICE or to any of its ingredients. (4)
WARNINGS AND PRECAUTIONS
- Severe Hypersensitivity: Permanently discontinue VIJOICE. Promptly initiate appropriate treatment. (5.1)
- Severe Cutaneous Adverse Reactions (SCARs): VIJOICE can cause SCARs, including Stevens-Johnson syndrome (SJS), erythema multiforme (EM), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS). Interrupt VIJOICE for signs or symptoms of SCARs. Permanently discontinue VIJOICE if SCARs are confirmed. (2.5, 5.2)
- Hyperglycemia: VIJOICE can cause severe hyperglycemia, in some cases associated with hyperglycemic hyperosmolar non-ketotic syndrome (HHNKS) or ketoacidosis. The safety of VIJOICE in patients with Type 1 or uncontrolled Type 2 diabetes has not been established. Before initiating treatment with VIJOICE, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment, monitor periodically. Initiate or optimize anti-hyperglycemic medications as clinically indicated. Interrupt, reduce dose, or discontinue VIJOICE if severe hyperglycemia occurs. (2.5, 5.3)
- Pneumonitis: VIJOICE can cause severe pneumonitis and interstitial lung disease. Monitor for clinical symptoms or radiological changes. Permanently discontinue VIJOICE if pneumonitis occurs. (2.5, 5.4)
- Diarrhea or Colitis: VIJOICE can cause severe diarrhea, resulting in dehydration and in some cases in acute kidney injury, as well as colitis. Monitor for diarrhea and additional symptoms of colitis, including abdominal pain and mucus or blood in stool. Interrupt, reduce dose, or permanently discontinue VIJOICE based on severity of diarrhea or colitis. Patients with colitis may require additional treatment, such as enteric-acting and/or systemic steroids. (2.5, 5.5)
- Embryo-Fetal Toxicity: VIJOICE can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (Grades 1 to 4, incidence ≥ 10%) were diarrhea, stomatitis, and hyperglycemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- CYP3A4 Inducers: Avoid coadministration of VIJOICE with a strong CYP3A4 inducer. Consider an alternative concomitant drug with no or minimal potential to induce CYP3A4. (7.1)
- Breast Cancer Resistance Protein (BCRP) Inhibitors: Avoid the use of BCRP inhibitors in patients treated with VIJOICE. If unable to use alternative drugs, closely monitor for increased adverse reactions. (7.1)
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 VIJOICE Dosage Form Overview
2.3 VIJOICE Administration Overview
2.4 VIJOICE Preparation and Administration Instructions
2.5 Dosage Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Hypersensitivity
5.2 Severe Cutaneous Adverse Reactions
5.3 Hyperglycemia
5.4 Pneumonitis
5.5 Diarrhea or Colitis
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience and Other Spontaneous Adverse Reaction Reports
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on VIJOICE
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VIJOICE is indicated for the treatment of adult and pediatric patients 2 years of age and older with severe manifestations of PIK3CA-Related Overgrowth Spectrum (PROS) who require systemic therapy.
This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Adult Patients
The recommended dosage of VIJOICE in adult patients is 250 mg orally, once daily, administered as recommended [see Dosage and Administration (2.2, 2.3, 2.4)] until disease progression or unacceptable toxicity.
Pediatric Patients (2 to less than 18 years of age)
The recommended initial dosage of VIJOICE in pediatric patients is 50 mg orally, once daily, administered as recommended [see Dosage and Administration (2.2, 2.3, 2.4)] until disease progression or unacceptable toxicity.
Consider a dose increase to 125 mg once daily in pediatric patients ≥ 6 years old for response optimization (clinical/radiological) after 24 weeks of treatment with VIJOICE at 50 mg once daily. When a pediatric patient turns 18 years old, consider a gradual dose increase up to 250 mg. Recommended dose increases by age group are listed in Table 1.
Table 1: Recommended Daily VIJOICE Dose Levels for Pediatric Patients (2 to less than 18 years of age) aDose can be administered as VIJOICE tablets or VIJOICE oral granules.
bA recommended increased dose has not been established.Patient age (years) Initial dose Dose increase 2 to < 6 50 mga Not applicableb 6 to < 18 50 mga 125 mg 2.2 VIJOICE Dosage Form Overview
VIJOICE is available in two dosage forms: tablets and oral granules.
Prescribe the most appropriate dosage form of VIJOICE according to the dose required and patient needs.
VIJOICE Tablets (50 mg, 125 mg, and 200 mg) may be administered as:
- Whole tablets: For patients who can swallow whole tablets.
- Tablets prepared as an oral suspension: For patients who have difficulty swallowing whole tablets [see Dosage and Administration (2.4)].
VIJOICE Oral Granules (50 mg per packet):
- For patients who are prescribed a 50 mg daily dose only [see Dosage and Administration (2.1)].
- Do not use multiple 50 mg packets or a partial packet of oral granules for patients prescribed a 125 mg or a 250 mg dose [see Clinical Pharmacology (12.3)].
- Do not combine VIJOICE tablets and VIJOICE oral granules to achieve the prescribed dose.
2.3 VIJOICE Administration Overview
Take VIJOICE with food at approximately the same time each day [see Clinical Pharmacology (12.3)].
If a dose of VIJOICE is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take VIJOICE at the usual time.
If the patient vomits after taking the dose, advise the patient not to take an additional dose on that day, and to resume the dosing schedule the next day at the usual time.
2.4 VIJOICE Preparation and Administration Instructions
VIJOICE Tablets
Swallow VIJOICE tablets whole, or prepare as a suspension to administer orally, or via feeding tubes.
VIJOICE Tablets, Whole
- Swallow VIJOICE tablets whole and take with food. Do not chew, divide, or crush.
- Do not use broken, cracked, or damaged tablets.
VIJOICE Tablets Prepared as a Suspension for Oral use, or Feeding Tubes (Nasogastric or Gastric Tube) Administration
-
For patients who are not able to swallow tablets, or who are using a feeding tube, prepare and administer VIJOICE as a suspension and take with food [see Clinical Pharmacology (12.3)].
- To prepare VIJOICE tablets as a suspension for oral use, place VIJOICE tablets in a cup containing 2 to 4 ounces of water. To prepare VIJOICE tablets as a suspension for feeding tubes administration, place VIJOICE tablets in a cup containing 1 to 2 ounces of water. Make the suspension with water only.
- Let tablets stand in water for approximately 5 minutes.
- Crush the tablets with a spoon and stir until a suspension is obtained.
- Immediately after preparation, administer the suspension as directed below. If not administered immediately after preparation, stir the suspension with the same spoon to re-suspend any particles before administration.
- Discard the suspension if it is not administered within 60 minutes after preparation.
Oral Administration
- Administer the suspension from the cup. After administration of the suspension, add approximately 1 to 2 ounces of water to the same cup. Stir with the same spoon to re-suspend any remaining particles and administer the entire contents of the cup orally. Repeat if particles remain.
Feeding Tubes Administration
- Administer VIJOICE tablets prepared as a suspension via French size 5 to 12 diameter silicone or polyurethane nasogastric tubes, or via French size 12 to 24 diameter silicone gastric tubes.
- Withdraw VIJOICE suspension from the cup into an enteral syringe and administer it via the nasogastric or gastric tube.
- After administration, add approximately 1 to 2 ounces (approximately 30 to 60 mL) of water to the same cup. Stir with the same spoon to re-suspend any remaining particles. Withdraw the contents of the cup into the same enteral syringe and administer it via the nasogastric or gastric tube. Repeat if particles remain.
VIJOICE Oral Granules
Administer VIJOICE oral granules directly onto the tongue with water, or prepare as a suspension or a mixture for oral use. To administer via feeding tubes prepare the suspension with water only.
- Each packet is for single use only.
- No packet should be used if the packet seal is broken.
- Do not attempt to use partial quantities of oral granules from 50 mg granules packets to prepare a dose. Do not combine VIJOICE tablets and VIJOICE oral granules to achieve the prescribed dose of 125 mg or 250 mg.
- For patients for whom a daily dose of 50 mg is prescribed, administer VIJOICE oral granules [see Clinical Pharmacology (12.3)] in one of the following ways:
VIJOICE Oral Granules for Direct Oral Administration
- Pour the contents of one VIJOICE oral granules packet directly onto the tongue and swallow with approximately 2 to 4 ounces of water. If needed, rinse the mouth with additional water and swallow to ensure no particles remain in the mouth.
VIJOICE Oral Granules as a Suspension or a Mixture for Oral administration
- Pour the contents of one VIJOICE oral granules packet into a cup.
- Add 1 to 3 teaspoons (about 0.5 ounces) of a beverage (water, milk, or apple juice) or soft food (applesauce or yogurt) and stir with a spoon, then administer the suspension or the mixture immediately.
- Rinse the cup with up to 2 ounces of a beverage (water, milk or apple juice) and administer the rinse immediately to ensure the entire dose is administered. If particles remain, repeat until the full dose is administered.
- If not administered immediately after preparation, stir the suspension or the mixture with the same spoon to re-suspend any particles before administration.
- Discard the oral granules mixed with water, milk, apple juice, applesauce, or yogurt if they are not administered within 60 minutes after preparation.
VIJOICE Oral Granules Suspension for Feeding Tubes Administration
For patients who are not able to swallow VIJOICE orally, administer VIJOICE via feeding tubes.
Administer VIJOICE granules via French size 8 to 12 diameter silicone or polyurethane nasogastric tubes or via French size 12 to 24 diameter silicone gastric tubes.
- Pour the contents of one VIJOICE granules packet into a cup.
- Add 4 teaspoons (about 0.7 ounces or 20 mL) of water and stir gently with a spoon until a suspension is obtained. Make the suspension with water only.
- Immediately after preparation, withdraw the suspension from the cup into an enteral syringe and administer it via the nasogastric or gastric tube.
- After administration, add 4 teaspoons (about 0.7 ounces or 20 mL) of water to the same cup. Stir with the same spoon to re-suspend any remaining particles. Withdraw the contents of the cup into the same enteral syringe and administer it via the nasogastric or gastric tube. Repeat if particles remain.
- If not administered immediately after preparation, stir the suspension with the same spoon to re-suspend any particles before withdrawing into an enteral syringe for administration.
- Discard the suspension if it is not administered within 60 minutes after preparation.
2.5 Dosage Modifications for Adverse Reactions
The recommended VIJOICE dose reductions for adverse reactions in adult and pediatric patients are listed in Table 2 and Table 3, respectively.
Table 2: VIJOICE Dosage Reduction Recommendations for Adverse Reactions in Adult Patients aDose can be administered as VIJOICE tablets or VIJOICE oral granules. VIJOICE dose level Dose and schedule First-dose reduction 125 mg once daily Second-dose reduction 50 mg once dailya Table 3: VIJOICE Dosage Reduction Recommendations for Adverse Reactions in Pediatric Patients aDose can be administered as VIJOICE tablets or VIJOICE oral granules. Action VIJOICE dose prior to dose reduction 125 mg once daily 50 mg once daily Dose reduction 50 mg once dailya Not applicable Discontinue VIJOICE in adults or pediatric patients who cannot tolerate 50 mg daily.
Tables 4, 5, 6, 7, 8, and 9 summarize recommendations for dose interruption, reduction, or discontinuation of VIJOICE in the management of specific adverse reactions.
Cutaneous Adverse Reactions
If a severe cutaneous adverse reaction (SCAR) is confirmed, permanently discontinue VIJOICE. Do not reintroduce VIJOICE in patients who have experienced previous SCAR during VIJOICE treatment [see Warnings and Precautions (5.2)].
Table 4: Dosage Modification and Management for Rash and Severe Cutaneous Adverse Reactions (SCARs) aGrading according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
bFor all grades of rash, consider consultation with a dermatologist.
cAntihistamines administered prior to rash onset may decrease incidence and severity of rash.[see Warnings and Precautions (5.1, 5.2)] Gradea,b Recommendation for adult and pediatric patientsc Grade 1
(< 10% body surface area [BSA] with active skin toxicity)No VIJOICE dosage modification is required unless the etiology is determined to be SCAR.
Initiate topical corticosteroid treatment.
Consider adding oral antihistamine to manage symptoms.
If active rash is not improved within 28 days of appropriate treatment, add a low dose systemic corticosteroid.
If the etiology is determined to be SCAR, permanently discontinue VIJOICE.Grade 2
(10% to 30% BSA with active skin toxicity)No VIJOICE dosage modification is required unless the etiology is determined to be SCAR.
Initiate or intensify topical corticosteroid and oral antihistamine treatment.
Consider low dose systemic corticosteroid treatment.
If rash improves to Grade ≤ 1 within 10 days, systemic corticosteroid may be discontinued.
If the etiology is determined to be SCAR, permanently discontinue VIJOICE.Grade 3 (e.g., severe rash not responsive to medical management)
(> 30% BSA with active skin toxicity)Interrupt VIJOICE and initiate or intensify topical/systemic corticosteroid and oral antihistamine treatment.
If the etiology is determined to be SCAR, permanently discontinue VIJOICE.
For rashes other than SCAR
Adult Patients:- Upon improvement to Grade ≤ 1, resume VIJOICE at the next lower dose level.
- Upon improvement to Grade ≤ 1, either resume VIJOICE at 50 mg while continuing oral antihistamine treatment or permanently discontinue VIJOICE.
- Permanently discontinue VIJOICE if:
- Patient was receiving antihistamines at the time of rash onset and antihistamine dose cannot be increased
- Grade ≥ 3 rash recurs
Grade 4 (e.g., severe bullous, blistering or exfoliating skin conditions)
(any % BSA associated with extensive superinfection, with IV antibiotics indicated; life-threatening consequences)Permanently discontinue VIJOICE. Hyperglycemia
Before initiating treatment with VIJOICE, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment with VIJOICE, monitor fasting glucose (FPG or fasting blood glucose) at least once every week for the first 2 weeks, then at least once every 4 weeks, and as clinically indicated. Monitor HbA1c every 3 months and as clinically indicated. In patients with risk factors for hyperglycemia, monitor fasting glucose more closely and as clinically indicated [see Warnings and Precautions (5.3)].
Table 5: Dosage Modification and Management for Hyperglycemia Abbreviation: ULN, upper limit of normal.
aFPG/Fasting Blood Glucose/Grade levels reflect hyperglycemia grading according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03.
bInitiate applicable anti-hyperglycemic medications, including metformin in adult and pediatric patients ≥ 10 years, SGLT2 inhibitors or insulin sensitizers (such as thiazolidinediones or dipeptidyl peptidase-4 inhibitors) in adult patients, and review respective prescribing information for dosing and dose titration recommendations, including local hyperglycemic treatment guidelines [see Warnings and Precautions (5.3)].[see Warnings and Precautions (5.3)] Fasting plasma glucose (FPG)/Fasting blood glucose valuesa Recommendation for adult and pediatric patients Dose modifications and management should only be based on fasting glucose values (FPG or fasting blood glucose). Grade 1
Fasting glucose > ULN -160 mg/dL or > ULN -8.9 mmol/LNo VIJOICE dosage modification is required.
Initiate or intensify oral anti-hyperglycemic treatmentb.Grade 2
Fasting glucose > 160 - 250 mg/dL or > 8.9 - 13.9 mmol/LNo VIJOICE dosage modification is required.
Initiate or intensify oral anti-hyperglycemic treatmentb.
Adult Patients:- If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days under appropriate anti-hyperglycemic treatmentb, reduce VIJOICE dose by 1 dose level and follow fasting glucose value-specific recommendations.
- If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days under appropriate anti-hyperglycemic treatmentb, interrupt VIJOICE until improvement to Grade ≤ 1, then resume VIJOICE at 50 mg and follow fasting glucose value-specific recommendations.
Grade 3
Fasting glucose > 250 - 500 mg/dL or > 13.9 - 27.8 mmol/LInterrupt VIJOICE.
Initiate or intensify oral anti-hyperglycemic treatmentb and consider additional anti-hyperglycemic medications for 1-2 days until hyperglycemia improves, as clinically indicated.
Administer intravenous hydration and consider appropriate treatment (e.g., intervention for electrolyte/ketoacidosis/hyperosmolar disturbances).
Adult Patients:- If fasting glucose decreases to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-hyperglycemic treatment, resume VIJOICE at 1 lower dose level.
- If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-hyperglycemic treatment, consultation with a physician with expertise in the treatment of hyperglycemia is recommended.
- If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days following appropriate anti-hyperglycemic treatmentb, permanently discontinue VIJOICE.
- If fasting glucose decreases to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-hyperglycemic treatment, resume VIJOICE at 50 mg.
- If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-hyperglycemic treatment, consultation with a physician with expertise in the treatment of hyperglycemia is recommended to determine if treatment with VIJOICE should be resumed or permanently discontinued.
- If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days following appropriate anti-hyperglycemic treatmentb, permanently discontinue VIJOICE.
- If hyperglycemia recurs at Grade ≥ 3, consider permanent discontinuation of VIJOICE.
Grade 4
Fasting glucose > 500 mg/dL or > 27.8 mmol/LInterrupt VIJOICE.
Initiate or intensify appropriate oral anti-hyperglycemic treatmentb.
Administer intravenous hydration and consider appropriate treatment (e.g., intervention for electrolyte/ketoacidosis/hyperosmolar disturbances).
Re-check fasting glucose within 24 hours and as clinically indicated.- If fasting glucose decreases to ≤ 500 mg/dL or 27.8 mmol/L, follow fasting glucose value-specific recommendations for Grade 3.
- If fasting glucose is confirmed at > 500 mg/dL or 27.8 mmol/L, permanently discontinue VIJOICE.
Pneumonitis
Table 6: Dosage Modification for Pneumonitis aGrading according to CTCAE Version 5.0. [see Warnings and Precautions (5.4)] Gradea Recommendation for adult and pediatric patients Any Grade Interrupt VIJOICE if pneumonitis is suspected.
Permanently discontinue VIJOICE if pneumonitis is confirmed.Diarrhea or Colitis
In pediatric patients, consider consultation with a physician with experience in the treatment of gastrointestinal conditions.
Table 7: Dosage Modification and Management for Diarrhea or Colitis aGrading according to CTCAE Version 5.0.
bFor Grade 2 and 3 colitis consider additional treatment, such as enteric-acting and/or systemic steroids.[see Warnings and Precautions (5.5)] Gradea Recommendation for adult and pediatric patients Grade 1 No VIJOICE dosage modification is required.
Initiate appropriate medical therapy and monitor as clinically indicated.Grade 2 Interrupt VIJOICE dose until improvement to Grade ≤ 1, then resume VIJOICE at the same dose level.
Initiate or intensify appropriate medical therapy and monitor as clinically indicatedb.
Adult Patients:- For recurrent Grade ≥ 2, interrupt VIJOICE dose until improvement to Grade ≤ 1, then resume VIJOICE at the next lower dose level.
- For recurrent Grade ≥ 2, interrupt VIJOICE dose until improvement to Grade ≤ 1, then resume VIJOICE at 50 mg.
Grade 3 Interrupt VIJOICE dose until improvement to Grade ≤ 1.
Initiate or intensify appropriate medical therapy and monitor as clinically indicatedb.
Adult Patients:- Once improved to Grade ≤ 1, then resume VIJOICE at the next lower dose level.
- Once improved to Grade ≤ 1, either resume VIJOICE at 50 mg or permanently discontinue VIJOICE.
- For recurrent Grade ≥ 3, consider permanent discontinuation of VIJOICE.
Grade 4 Permanently discontinue VIJOICE. Pancreatitis
Table 8: Dosage Modification for Pancreatitis aGrading according to CTCAE Version 5.0. Gradea Recommendation for adult and pediatric patients Grade 2 Interrupt VIJOICE dose until improvement to Grade < 2.
Adult Patients:- Resume VIJOICE at the next lower dose level (only one dose reduction is permitted).
- If pancreatitis recurs, permanently discontinue VIJOICE.
- Resume VIJOICE at 50 mg.
- If pancreatitis recurs, permanently discontinue VIJOICE.
Grade 3 Adult Patients: - Interrupt VIJOICE dose until improvement to Grade < 2.
- Resume VIJOICE at the next lower dose level (only one dose reduction is permitted).
- If pancreatitis recurs, permanently discontinue VIJOICE.
- Permanently discontinue VIJOICE.
Grade 4 Permanently discontinue VIJOICE. Other Adverse Reactions
Table 9: Dosage Modification and Management for Other Adverse Reactions (Excluding Rash and Severe Cutaneous Adverse Reactions, Hyperglycemia, Pneumonitis, Diarrhea or Colitis, and Pancreatitis) aGrading according to CTCAE Version 5.0.
bFor Grade 2 total bilirubin elevation in adult patients, interrupt VIJOICE dose until improvement to Grade ≤ 1. If improvement occurs in ≤ 14 days, resume at the same dose level. If improvement occurs in > 14 days, resume VIJOICE at the next lower dose level.
cFor Grade 2 total bilirubin elevation in pediatric patients, interrupt VIJOICE dose until improvement to Grade ≤ 1. If improvement occurs in ≤ 14 days, resume at the same dose level. If improvement occurs in > 14 days, resume VIJOICE at 50 mg.
dIf alopecia becomes a concern, consider consulting a dermatologist.Gradea Recommendation for adult and pediatric patients Grade 1 or 2b,c,d No VIJOICE dosage modification is required.
Initiate appropriate medical therapy and monitor as clinically indicatedb,c,d.Grade 3 Interrupt VIJOICE dose until improvement to Grade ≤ 1.
Initiate or intensify appropriate medical therapy and monitor as clinically indicated.
Adult Patients:- Once improved to Grade ≤ 1, then resume VIJOICE at the next lower dose level.
- Once improved to Grade ≤ 1, either resume VIJOICE at 50 mg or permanently discontinue VIJOICE.
- If adverse reaction recurs at Grade ≥ 3, consider permanent discontinuation of VIJOICE.
- Consider consultation with a qualified physician with specific expertise in the field of the concerned adverse reaction.
Grade 4 Permanently discontinue VIJOICE. -
3 DOSAGE FORMS AND STRENGTHS
Tablets
50 mg: Light yellow, unscored, round and curved with beveled edges film-coated tablet, debossed with “C7” on one side and “NVR” on the other side.
125 mg: Dark yellow, unscored, ovaloid and curved with beveled edges film-coated tablet, debossed with “Y7” on one side and “NVR” on the other side.
200 mg: Pale yellow, unscored, ovaloid and curved with beveled edges film-coated tablet, debossed with “CL7” on one side and “NVR” on the other side.
Oral Granules
50 mg: White to almost white free flowing mixture of powder and granules in packets.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Hypersensitivity
Severe hypersensitivity reactions, including anaphylaxis, anaphylactic shock, and angioedema, have occurred in adult patients treated with alpelisib in the oncology setting and in patients treated with VIJOICE [see Adverse Reactions (6.1)]. VIJOICE is not approved for use in the oncology setting.
Permanently discontinue VIJOICE in the event of severe hypersensitivity [see Contraindications (4)].
5.2 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS), erythema multiforme (EM), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS), have occurred in adult patients treated with alpelisib in the oncology setting and may occur in patients treated with VIJOICE. VIJOICE is not approved for use in the oncology setting.
If signs or symptoms of SCARs occur, interrupt VIJOICE until the etiology of the reaction has been determined. Consultation with a dermatologist is recommended.
If a SCAR is confirmed, permanently discontinue VIJOICE.
If a SCAR is not confirmed, VIJOICE may require dose modifications, topical or systemic corticosteroids, or oral antihistamine treatment as described in Table 4 [see Dosage and Administration (2.5)].
5.3 Hyperglycemia
Severe hyperglycemia, in some cases associated with hyperglycemic hyperosmolar non-ketotic syndrome (HHNKS) or fatal cases of ketoacidosis, has occurred in adult patients treated with alpelisib in the oncology setting and may occur in patients treated with VIJOICE. VIJOICE is not approved for use in the oncology setting.
In the EPIK-P1 study, Grade 1 or 2 hyperglycemia was reported in 12% of patients treated with VIJOICE [see Adverse Reactions (6.1)].
Before initiating treatment with VIJOICE, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment with VIJOICE, monitor fasting glucose (FPG or fasting blood glucose) at least once every week for the first 2 weeks, then at least once every 4 weeks, and as clinically indicated. Monitor HbA1c every 3 months and as clinically indicated. Monitor fasting glucose more frequently for the first few weeks during treatment with VIJOICE in patients with risk factors for hyperglycemia, such as obesity (BMI ≥ 30), elevated FPG, HbA1c at the upper limit of normal or above, use of concomitant systemic corticosteroids, or age ≥ 75 [see Use in Specific Populations (8.5)].
If a patient experiences hyperglycemia after initiating treatment with VIJOICE, monitor fasting glucose as clinically indicated, and at least twice weekly until fasting glucose decreases to normal levels. During treatment with anti-hyperglycemic medication, continue monitoring fasting glucose at least once a week for 8 weeks, followed by once every 2 weeks and as clinically indicated. Consider consultation with a healthcare practitioner with expertise in the treatment of hyperglycemia and counsel patients on lifestyle changes.
The safety of VIJOICE in patients with Type 1 and uncontrolled Type 2 diabetes has not been established. Patients with a history of diabetes mellitus may require intensified hyperglycemic treatment. Closely monitor patients with diabetes.
Interrupt, reduce the dose, or permanently discontinue VIJOICE based on the severity as described in Table 5 [see Dosage and Administration (2.5)].
5.4 Pneumonitis
Severe pneumonitis, including acute interstitial pneumonitis and interstitial lung disease, has occurred in adult patients treated with alpelisib in the oncology setting and may occur in patients treated with VIJOICE. VIJOICE is not approved for use in the oncology setting.
In patients who have new or worsening respiratory symptoms or are suspected to have developed pneumonitis, interrupt VIJOICE immediately and evaluate the patient for pneumonitis. Consider a diagnosis of non-infectious pneumonitis in patients presenting with non-specific respiratory signs and symptoms, such as hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations.
Permanently discontinue VIJOICE in all patients with confirmed pneumonitis [see Dosage and Administration (2.5)].
5.5 Diarrhea or Colitis
Severe diarrhea, resulting in dehydration and in some cases in acute kidney injury, as well as colitis, have occurred in adult patients treated with alpelisib in the oncology setting and may occur in patients treated with VIJOICE. VIJOICE is not approved for use in the oncology setting.
In the EPIK-P1 study, 16% of patients experienced Grade 1 diarrhea during treatment with VIJOICE [see Adverse Reactions (6.1)].
Monitor patients for diarrhea and additional symptoms of colitis, such as abdominal pain and mucus or blood in stool. Interrupt, reduce the dose or permanently discontinue VIJOICE based on the severity of diarrhea or colitis [see Dosage and Administration (2.5)].
Patients with colitis may require additional treatment, such as enteric-acting and/or systemic steroids [see Dosage and Administration (2.5)].
5.6 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, VIJOICE can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of alpelisib to pregnant animals during organogenesis caused adverse developmental outcomes, including embryo-fetal mortality (post-implantation loss), reduced fetal weights, and increased incidences of fetal malformations at doses that were approximately equivalent to the recommended pediatric and adult doses. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with VIJOICE and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with VIJOICE and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Severe Hypersensitivity [see Warnings and Precautions (5.1)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.2)]
- Hyperglycemia [see Warnings and Precautions (5.3)]
- Pneumonitis [see Warnings and Precautions (5.4)]
- Diarrhea or Colitis [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of VIJOICE was evaluated in EPIK-P1 (NCT04285723), a single-arm clinical study in patients who were treated as part of an expanded access program for compassionate use. Fifty-seven patients 2 years of age and older with severe or life-threatening PIK3CA-Related Overgrowth Spectrum (PROS) received VIJOICE based on age at dosages ranging from 50 mg to 250 mg orally once daily [see Clinical Studies (14)]. Among patients who received VIJOICE, 95% were exposed for 6 months or longer and 79% were exposed for greater than one year.
The median age of patients who received VIJOICE was 14 years (range, 2 to 50); 58% were female; 12% were White and race was not reported for 88%.
Serious adverse reactions occurred in 12% of patients who received VIJOICE. Serious adverse reactions occurring in two or more patients included dehydration (n = 2) and cellulitis (n = 2).
Dosage interruption of VIJOICE due to an adverse reaction occurred in 11% of patients. Adverse reactions which required dosage interruption in two or more patients included dizziness (n = 2) and vomiting (n = 2). Dose reductions of VIJOICE due to an adverse reaction occurred in 5% of patients. Adverse reactions which required dose reduction included alopecia, memory impairment, and soft tissue infection.
The most common adverse reactions (≥ 10%) were diarrhea, stomatitis, and hyperglycemia. The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were increased glucose, decreased hemoglobin, decreased phosphate, increased bilirubin, decreased sodium, and decreased platelets.
Adverse reactions and laboratory abnormalities are listed in Table 10 and Table 11, respectively.
Table 10: Adverse Reactions (≥ 5%) in Patients with PROS Who Received VIJOICE in EPIK-P1 Grading according to CTCAE Version 4.03.
aStomatitis: including stomatitis and aphthous ulcer.VIJOICE
N = 57Adverse reactions All Grades
(%)Grade 3 or 4
(%)Gastrointestinal disorders Diarrhea 16 0 Stomatitisa 16 0 Metabolism and nutrition disorders Hyperglycemia 12 0 Skin and subcutaneous tissue disorders Eczema 7 0 Dry skin 7 0 Alopecia 5 0 Nervous system disorders Headache 5 0 Infections and infestations Cellulitis 5 3.5 Clinically relevant adverse reactions in < 5% of patients who received VIJOICE included nausea, vomiting, dehydration, and mucosal dryness.
Table 11: Laboratory Abnormalities Worsening from Baseline in ≥ 10% of Patients with PROS Who Received VIJOICE in EPIK-P1 Grading according to CTCAE Version 4.03.
Abbreviation: N/A, not available.
aThe denominator used to calculate the rate varied from 9 to 50 based on the number of patients with a baseline value and at least one post-treatment value.
bNo Grade 4 laboratory abnormalities were reported.
cGlucose increase is an expected laboratory abnormality of PI3K inhibition.
dNo CTCAE grade available. For HbA1c, baseline values increasing post-treatment to a value above the upper limit of the normal range (≥ 5.7%) are considered increased.Laboratory abnormality VIJOICEa
N = 57All Grades
%Grade 3 or 4
%Chemistry Decreased calcium (corrected) 60 0 Decreased phosphate 59 5b Increased glucosec 56 11b Increased glycosylated hemoglobin (HbA1c)d 38d N/Ad Increased creatinine 31 0 Increased bilirubin 29 2b Increased potassium 24 0 Increased triglycerides 19 0 Decreased magnesium 18 0 Increased aspartate aminotransferase (AST) 17 0 Increased cholesterol 13 0 Decreased albumin 13 0 Decreased sodium 12 2b Decreased potassium 12 0 Increased gamma glutamyl transferase (GGT) 11 0 Increased alanine aminotransferase (ALT) 10 0 Hematology Decreased leukocyte 22 0 Decreased hemoglobin 20 6b Decreased lymphocyte 20 0 Decreased neutrophil 19 0 Increased lymphocyte 17 0 Decreased platelets 14 2b Other Clinical Trials Experience
The following additional adverse reactions and laboratory abnormality have been identified following administration of VIJOICE: hypersensitivity, lipase increased, dermatitis, and abdominal pain.
6.2 Postmarketing Experience and Other Spontaneous Adverse Reaction Reports
The following adverse reactions have been identified with VIJOICE use in patients with PROS in an expanded access program for compassionate use. Because these reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Metabolism and nutrition disorders: Decreased appetite.
Skin and subcutaneous tissue disorders: Pruritus, rash (including rash maculo-papular, rash erythematous, rash papular, and rash pruritic), acne (including dermatitis acneiform).
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on VIJOICE
CYP3A4 Inducers
Avoid coadministration of VIJOICE with strong CYP3A4 inducers and consider an alternative concomitant drug with no or minimal potential to induce CYP3A4.
Alpelisib is metabolized by CYP3A4. Concomitant use of VIJOICE with a strong CYP3A4 inducer may decrease alpelisib concentration [see Clinical Pharmacology (12.3)], which may decrease alpelisib activity.
Breast Cancer Resistance Protein Inhibitors (BCRP)
Avoid the use of BCRP inhibitors in patients treated with VIJOICE. If unable to use alternative drugs, when VIJOICE is used in combination with BCRP inhibitors, closely monitor for increased adverse reactions.
Alpelisib is transported by BCRP. Concomitant use of VIJOICE with a BCRP inhibitor may increase alpelisib exposure [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data and mechanism of action, VIJOICE can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, oral administration of alpelisib to pregnant rats and rabbits during organogenesis caused adverse developmental outcomes, including embryo-fetal mortality (post-implantation loss), reduced fetal weights, and increased incidences of fetal malformations at doses described below (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. However, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies in the U.S. general population.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals received oral doses of alpelisib during the period of organogenesis. In the rat study, animals were dosed at 3, 10, or 30 mg/kg/day from gestation day 6 to 17; and in the rabbit study, animals were dosed at 3, 15, 25, and 30 mg/kg/day from gestation day 7 to 20.
In rats, oral administration of alpelisib resulted in maternal toxicity (body weight loss, low food consumption) and no viable fetuses (post-implantation loss) at 30 mg/kg/day (approximately 3.6 to 1.2 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA). At a dose of 10 mg/kg/day (approximately 1.2 to 0.4 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA), toxicities included reduced fetal weight and increased incidences of skeletal malformations (bent scapula and thickened or bent long bones) and fetal variations (enlarged brain ventricle, decreased bone ossification).
In a pilot embryo-fetal development study in rabbits, a dose of 30 mg/kg/day (approximately 7 to 2.2 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients based on BSA) resulted in no viable fetuses (post-implantation loss). Doses ≥ 15 mg/kg/day (approximately 3.5 to 1.1 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA) resulted in increased embryo-fetal deaths, reduced fetal weights, and malformations, mostly related to the tail and head.
8.2 Lactation
Risk Summary
There are no data on the presence of alpelisib in human milk, its effects on milk production, or the breastfed child. Because of the potential for serious adverse reactions in the breastfed child, advise lactating women to not breastfeed during treatment with VIJOICE and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status in females of reproductive potential prior to initiating VIJOICE.
Contraception
Females
VIJOICE can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with VIJOICE and for 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with VIJOICE and for 1 week after the last dose.
Infertility
Based on findings from animal studies, VIJOICE may impair fertility in males and females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of VIJOICE have been established in pediatric patients 2 to less than 18 years of age with PROS based on results from a single-arm clinical study of VIJOICE (EPIK-P1) that enrolled 39 pediatric patients: 11 patients aged 2 to 5 years, 12 patients aged 6 to 11 years, and 16 patients aged 12 to less than 18 years of age [see Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of VIJOICE in pediatric patients below the age of 2 years have not been established.
Although there were no new safety signals observed in pediatric patients, there is insufficient data to determine whether VIJOICE has an adverse impact on growth and development in pediatric patients with PROS. Based on the animal toxicity data (described below), regular monitoring of growth and development in pediatric patients treated with VIJOICE is recommended.
Animal Toxicity Data
In a 4-week general toxicology study, rats administered alpelisib had growth plate thickening and decreased trabeculae of the knee joint, dentin thinning, and degenerative odontoblasts at the dose of 30 mg/kg/day (approximately 2.8 to 1.2 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA). Dentin thinning/irregular dentin was also observed in the 13-week toxicology study in rats at the high dose of 20 mg/kg/day (approximately 2 to 0.8 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA).
-
10 OVERDOSAGE
There is limited experience of overdose with alpelisib in clinical trials.
In cases where accidental overdosage of alpelisib was reported in the clinical studies, the adverse reactions associated with the overdose were consistent with the known safety profile of alpelisib and included hyperglycemia, nausea, asthenia, and rash.
Initiate general symptomatic and supportive measures in all cases of overdosage where necessary. There is no known antidote for VIJOICE.
-
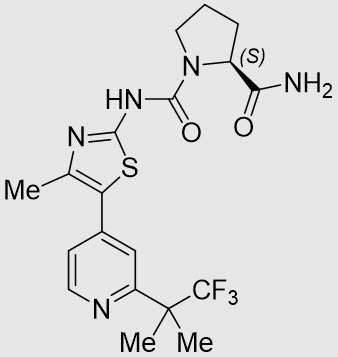
11 DESCRIPTION
VIJOICE (alpelisib) is a kinase inhibitor. The chemical name of alpelisib is (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide. Alpelisib is a white to almost white powder. The molecular formula for alpelisib is C19H22F3N5O2S and the relative molecular mass is 441.47 g/mol. The pH of a 1.0% (m/V) solution of alpelisib in water/ethanol (50:50 V/V) is approximately 6.2. The chemical structure of alpelisib is shown below:
VIJOICE film-coated tablets are supplied for oral administration with three strengths that contain 50 mg, 125 mg, and 200 mg of alpelisib. The tablets also contain hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate. The film-coating contains hypromellose, iron oxide red (applicable only to 50 mg and 200 mg strengths), iron oxide yellow, macrogol/polyethylene glycol (PEG) 4000, talc, and titanium dioxide.
VIJOICE oral granules are supplied for oral administration with one strength that contains 50 mg of alpelisib. The granules also contain hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Alpelisib is an inhibitor of phosphatidylinositol-3-kinase (PI3K) with inhibitory activity predominantly against PI3Kα. Gain-of-function mutations in the gene encoding the catalytic α-subunit of PI3K (PIK3CA) lead to activation of PI3Kα and Akt-signaling, cellular transformation and the generation of tumors in in vitro and in vivo models.
Activating mutations in PIK3CA have been found to induce a spectrum of overgrowths and malformations comprising a wide group of clinically recognizable disorders commonly known as PROS.
In an inducible mouse model of Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevi, Scoliosis/Skeletal and Spinal syndrome (CLOVES), a phenotype of PROS, alpelisib inhibition of the PI3K pathway resulted in the prevention or improvement of organ abnormalities associated with the disease, depending on when alpelisib treatment was started. These findings were reversed after withdrawal of alpelisib.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of VIJOICE have not been characterized.
Cardiac Electrophysiology
At a dose of 300 mg, alpelisib does not prolong the QT interval to any clinically relevant extent in the oncology setting. VIJOICE is not approved for use in the oncology setting.
12.3 Pharmacokinetics
The pharmacokinetics of alpelisib has been studied in healthy subjects and adult patients with solid tumors and are presented as mean (% CV) under fed conditions unless otherwise specified. Alpelisib maximum plasma concentration (Cmax) was 277 ng/mL (24%) and area under the curve (AUC) was 2,090 hr*ng/mL (24%) following administration of a single 50 mg dose of VIJOICE. Steady state alpelisib Cmax and AUC increased proportionally over the dose range of 30 mg (0.6 times the lowest approved recommended dosage) to 450 mg (1.8 times the highest approved recommended dosage) under fed conditions. The mean accumulation of alpelisib was 1.3 to 1.5 and steady state plasma concentrations were reached within 3 days following daily dosage.
Absorption
The median time to reach peak plasma concentration (Tmax) ranged between 2.0 to 4.0 hours.
Effect of Food
A high-fat high-calorie (HFHC) meal (985 calories with 58.1 g of fat) increased alpelisib AUC by 73% and Cmax by 84%, and a low-fat low-calorie (LFLC) meal (334 calories with 8.7 g of fat) increased alpelisib AUC by 77% and Cmax by 145% following administration of a single 300 mg dose of alpelisib as tablets. No clinically relevant differences in alpelisib AUC were observed between LFLC and HFHC meals.
No clinically relevant food effect on alpelisib pharmacokinetics was observed after a single 50 mg oral dose of VIJOICE as granules taken with a LFLC meal as compared to that taken under fasted conditions.
No clinically relevant differences in alpelisib Cmax and AUC were observed following administration of a single 50 mg dose of VIJOICE as granules or tablets taken with a LFLC meal.
Distribution
The apparent volume of distribution of alpelisib at steady state is 114 L (46%). Protein binding of alpelisib is 89% and is independent of concentration.
Elimination
The half-life of alpelisib is predicted to be 8 to 9 hours. The clearance of alpelisib is 9.2 L/hr (21%) under fed conditions.
Metabolism
Alpelisib is primarily metabolized by chemical and enzymatic hydrolysis to form its metabolite BZG791 and followed by CYP3A4 mediated hydroxylation.
Excretion
Following a single oral dose of 400 mg (1.6 times the highest approved recommended dosage) radiolabeled alpelisib under fasted condition, 81% of the administered dose was recovered in feces (36% unchanged) and 14% (2% unchanged) in urine. CYP3A4-mediated metabolites (12%) and glucuronides amounted to approximately 15% of the dose.
Specific Populations
No clinically significant differences in the pharmacokinetics of alpelisib were predicted based on age (21 to 87 years), sex, race/ethnicity (Japanese or Caucasian), body weight (37 to 181 kg), mild to moderate renal impairment (CLcr 30 to < 90 mL/min based on the Cockcroft-Gault formula), or mild to severe hepatic impairment (Child-Pugh Class A, B, and C). The effect of severe renal impairment (CLcr < 30 mL/min) on the pharmacokinetics of alpelisib is unknown.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Acid Reducing Agents: No clinically significant differences in the pharmacokinetics of alpelisib were observed when used concomitantly with ranitidine (H2 receptor antagonist) and administered with food as directed.
Concomitant use of ranitidine decreased alpelisib AUC approximately 30% and Cmax by 51% with a single 300 mg oral dose (1.2 times the highest approved recommended dosage) of alpelisib under the fasted state. In the presence of a low-fat low-calorie meal, AUC was decreased by 21% and Cmax by 36% with ranitidine.
CYP3A4, CYP2C8, CYP2C9, CYP2C19 and CYP2B6 Substrates: Coadministration of repeated doses of alpelisib 300 mg with a single-dose of sensitive substrates of CYP3A4 (midazolam), CYP2C8 (repaglinide), CYP2C9 (warfarin), CYP2C19 (omeprazole) and CYP2B6 (bupropion), administered as a cocktail did not show clinically significant pharmacokinetic interactions.
No clinically significant differences in pharmacokinetics of everolimus (a substrate of CYP3A4 and P-gp) were observed when used concurrently with alpelisib.
Effect of CYP3A4 Inducers on Alpelisib: Coadministration of repeat doses of rifampin (a strong CYP3A4 inducer) with a single 300 mg dose of alpelisib decreased alpelisib Cmax by 38% and AUC by 57%, respectively. Coadministration of rifampin with repeat doses of 300 mg alpelisib decreased alpelisib Cmax by 59% and AUC by 74%, respectively.
Model-Informed Approaches
Coadministration of repeat doses of ketoconazole (a strong CYP3A4 inhibitor) with a single 300 mg dose of alpelisib is expected to increase alpelisib AUC by 37% or less.
Coadministration of repeat doses of efavirenz (a moderate CYP3A4 inducer) with a single 300 mg dose of alpelisib is expected to decrease alpelisib AUC by 30% or less.
In Vitro Studies
Effect of Transporter on Alpelisib: Alpelisib is a substrate of BCRP.
Effect of Alpelisib on Transporters: Alpelisib is an inhibitor of P-gp. Alpelisib has a low potential to inhibit BCRP, MRP2, BSEP, OATP1B1, OATP1B3, OCT1, OAT1, OAT3, OCT2, MATE1, and MATE2K at clinically relevant concentrations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Alpelisib was not carcinogenic in a 2-year carcinogenicity study conducted in rats when administered by daily oral gavage at doses up to 4 mg/kg (approximately 0.5 to 0.2 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA).
Alpelisib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay, or aneugenic or clastogenic in human cell micronucleus and chromosome aberration tests. Alpelisib was not genotoxic in an in vivo rat micronucleus test.
In a fertility and early embryonic development study in rats, female animals were administered alpelisib doses of 3, 10, and 20 mg/kg/day orally. Animals were dosed for 4-weeks prior to pairing, during the mating period, and up to Gestation Day 6. At the dose of 20 mg/kg/day, alpelisib increased pre- and post-implantation losses, leading to reduced numbers of implantation sites and live embryos. These findings were observed at doses approximately 2.4 to 0.8 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on body surface areas (BSA). In a repeated-dose toxicity study in rats, adverse effects in female reproductive organs included vaginal atrophy and estrous cycle variations in rats at doses ≥ 6 mg/kg/day (approximately 0.7 to 0.2 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA).
In a male fertility study, alpelisib administered orally at doses of 3, 10 and 20 mg/kg/day for up to 99 days (10-weeks prior to pairing, during mating period and continuing during post-pairing) to male rats, resulted in reduced weights of seminal vesicles and prostate, which correlated with atrophy and/or reduced secretion in prostate and seminal vesicles at ≥ 10 mg/kg/day (approximately 1.2 to 0.4 times the initial recommended doses of 50 mg and 250 mg in pediatric and adult patients, respectively, based on BSA). No adverse effects on male fertility parameters were observed at doses up to 20 mg/kg/day.
-
14 CLINICAL STUDIES
The efficacy of VIJOICE was assessed in EPIK-P1 (NCT04285723), a single-arm clinical study in patients who were treated as part of an expanded access program for compassionate use which enrolled patients across seven sites in five countries (France, Spain, US, Ireland, and Australia). Eligible patients 2 years of age and older with PIK3CA-Related Overgrowth Spectrum (PROS) who received VIJOICE had clinical manifestations of PROS that were assessed by the treating physician as severe or life-threatening and necessitating systemic treatment and had documented evidence of mutation in the PIK3CA gene. Patients received VIJOICE at dosages based on age ranging from 50 mg to 250 mg orally once daily.
The efficacy of VIJOICE was evaluated in a total of 37 patients with at least one target lesion identified on imaging performed within 24 weeks prior to receipt of the first dose of VIJOICE. The median age of patients was 14 years (range: 2 to 38); 22% of patients were 2 to 5 years, 22% were 6 to 11 years, 27% were 12 to less than 18 years of age, and 30% were ≥ 18 years; 57% were female, 11% were White and race was not reported for 89%. Ninety-two percent of patients had congenital overgrowth and 8% had early childhood-onset. Patients had heterogeneous manifestations of PROS, including CLOVES (81%), Megalencephaly-Capillary Malformation Polymicrogyria (MCAP; 8%), Klippel-Trenaunay Syndrome (KTS; 2.7%), Facial Infiltrating Lipomatosis (FIL; 8%), and Other (5%). Five percent (5%) of patients had concurrent manifestations of CLOVES and MCAP.
The major efficacy outcome measure for the study was the proportion of patients with radiological response at Week 24 as determined by blinded independent central review (BICR), defined as a ≥ 20% reduction from baseline in the sum of measurable target lesion volume (1 to 3 lesions) confirmed by at least one subsequent imaging assessment, in the absence of a ≥ 20% increase from baseline in any target lesion, progression of non-target lesions, or appearance of a new lesion. An additional efficacy outcome measure was duration of response, defined as the time from the first documented response to the date of the first documented disease progression or death due to any cause.
Efficacy results are presented in Table 12.
Table 12: Efficacy Results at Week 24 in EPIK-P1 Abbreviation: +, censored observation.
aConfirmed response as determined by blinded independent central review (BICR).
bPatients without any response assessment at Week 24 were considered non-responders.Efficacy parameters All patients
N = 37Response ratea,b Responders, n (%)
95% CI10 (27)
(14, 44)Duration of response (DOR) Median in months (range) NR (0.9+, 42.9+) % ≥ 6 months 70 % ≥ 12 months 60 -
16 HOW SUPPLIED/STORAGE AND HANDLING
VIJOICE (alpelisib) 50 mg, 125 mg, and 200 mg film-coated tablets are available in blister packs based on daily dose as described in Table 13.
Table 13: VIJOICE Tablets Daily Dose Blister Packs Daily dose Each carton contains Each child-resistant blister pack contains Appearance of tablet NDC 50 mg daily dose One 28-day supply blister pack 28 tablets: 50 mg alpelisib per tablet Light yellow, unscored, round and curved with beveled edges, debossed with “C7” on one side and “NVR” on the other side NDC: 0078-1021-84 125 mg daily dose One 28-day supply blister pack 28 tablets: 125 mg alpelisib per tablet Dark yellow, unscored, ovaloid and curved with beveled edges, debossed with “Y7” on one side and “NVR” on the other side NDC: 0078-1028-84 250 mg daily dose Two 14-day supply blister packs (56 tablets total) 14 tablets: 200 mg alpelisib per tablet, and
14 tablets: 50 mg alpelisib per tabletPale yellow, unscored, ovaloid and curved with beveled edges, debossed with “CL7” on one side and “NVR” on the other side
Light yellow, unscored, round and curved with beveled edges, debossed with “C7” on one side and “NVR” on the other sideNDC: 0078-1035-02 Table 14: VIJOICE Oral Granules Daily dose Each carton contains Each child-resistant packet contains Appearance NDC 50 mg daily dose 28 packets (28-day supply of single-use packets) 50 mg alpelisib White to almost white free flowing mixture of powder and granules NDC: 0078-1175-51 Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and their caregivers to read the FDA-approved patient labeling (Patient Information).
Severe Hypersensitivity
Inform patients and their caregivers of the signs and symptoms of hypersensitivity. Advise patients and their caregivers to contact their healthcare provider immediately for signs and symptoms of hypersensitivity [see Warnings and Precautions (5.1)].
Severe Cutaneous Adverse Reactions
Inform patients and their caregivers of the signs and symptoms of severe cutaneous adverse reactions (SCARs). Advise patients and their caregivers to contact their healthcare provider immediately for signs and symptoms of SCARs (e.g., a prodrome of fever, flu-like symptoms, mucosal lesions, progressive skin rash, or lymphadenopathy) [see Warnings and Precautions (5.2)].
Hyperglycemia
Advise patients and their caregivers that VIJOICE may cause hyperglycemia and the need to monitor fasting glucose periodically during therapy. Advise patients and their caregivers of the signs and symptoms of hyperglycemia (e.g., excessive thirst, urinating more often than usual or higher amount of urine than usual, or increased appetite with weight loss) [see Warnings and Precautions (5.3)].
Pneumonitis
Inform patients and their caregivers that VIJOICE may cause pneumonitis and to immediately report new or worsening respiratory symptoms [see Warnings and Precautions (5.4)].
Diarrhea or Colitis
Advise patients and their caregivers that VIJOICE may cause diarrhea, which may be severe, and to start anti-diarrheal treatment, increase oral fluids, and notify their healthcare provider if diarrhea occurs while taking VIJOICE [see Warnings and Precautions (5.5)].
Advise patients and their caregivers that VIJOICE may cause colitis and to notify their healthcare provider immediately of any symptoms of colitis, such as abdominal pain and mucus or blood in stool, while taking VIJOICE [see Warnings and Precautions (5.5)].
Alopecia
Advise patients and caregivers that VIJOICE may cause alopecia [see Adverse Reactions (6.1)]. Advise patients and caregivers to contact a dermatologist if alopecia becomes a concern [see Dosage and Administration (2.5)].
Embryo-Fetal Toxicity
- Inform pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with VIJOICE and for 1 week after the last dose [see Use in Specific Populations (8.3)].
- Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with VIJOICE and for 1 week after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with VIJOICE and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential that VIJOICE may impair fertility [see Use in Specific Populations (8.3)].
Drug Interactions
Advise patients and their caregivers to inform their healthcare providers of all concomitant medications, herbal and dietary supplements [see Drug Interactions (7.1)].
Dosing
Instruct patients and their caregivers of the following:
- Take VIJOICE with food at approximately the same time each day [see Dosage and Administration (2.3)].
- Swallow the tablets whole (tablets should not be crushed, chewed, or split prior to swallowing) [see Dosage and Administration (2.4)].
- For patients unable to swallow tablets, advise how to prepare an oral suspension using the tablets or how to take the oral granules (if applicable) [see Dosage and Administration (2.4)].
- If a dose of VIJOICE is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take VIJOICE at the usual time. Instruct patients not to take 2 doses to make up for a missed dose [see Dosage and Administration (2.3)].
- If vomiting occurs after taking the dose of VIJOICE, they should not take an additional dose on that day and should resume the usual dosing schedule the next day at the usual time [see Dosage and Administration (2.3)].
Distributed by
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936© Novartis
T2025-38
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 7/2025 PATIENT INFORMATION VIJOICE® (vi’ joiz)
(alpelisib)
tabletsVIJOICE® (vi’ joiz)
(alpelisib)
oral granulesWhat is VIJOICE?
VIJOICE is a prescription medicine used to treat adults and children 2 years of age and older with severe phosphatidylinositol-3-kinase catalytic subunit alpha (PIK3CA)-Related Overgrowth Spectrum (PROS).
It is not known if VIJOICE is safe and effective in children below 2 years of age.Do not take VIJOICE if you have had a severe allergic reaction to alpelisib or are allergic to any of the ingredients in VIJOICE. - See the end of this Patient Information leaflet for a complete list of the ingredients in VIJOICE.
- See “What are the possible side effects of VIJOICE?” for signs and symptoms of severe allergic reactions.
Before you take VIJOICE, tell your healthcare provider about all of your medical conditions, including if you: - have a history of diabetes.
- have a history of skin rash, redness of skin, blistering of the lips, eyes or mouth, or skin peeling.
- are pregnant or plan to become pregnant. VIJOICE can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before you start treatment with VIJOICE.
- You should use effective birth control (contraception) during treatment with VIJOICE and for 1 week after the last dose. Talk to your healthcare provider about birth control methods that may be right for you during this time.
- If you become pregnant or think you are pregnant, tell your healthcare provider right away.
- are breastfeeding or plan to breastfeed. It is not known if VIJOICE passes into your breast milk. Do not breastfeed during treatment with VIJOICE and for 1 week after the last dose.
How should I take VIJOICE? - Read the detailed “Instructions for Use” that comes with VIJOICE for information about the right way to prepare and take or give a dose of VIJOICE tablets or oral granules.
- Take VIJOICE exactly as your healthcare provider tells you.
- Do not change your dose or stop taking VIJOICE unless your healthcare provider tells you.
- Take VIJOICE 1 time each day, at about the same time each day.
- Take VIJOICE with food.
- VIJOICE comes as tablets and oral granules. Your healthcare provider will prescribe the dosage form that is right for you:
- If your prescribed daily dose is 50 mg, your healthcare provider may prescribe VIJOICE tablets or VIJOICE oral granules.
- If your prescribed daily dose is 125 mg or 250 mg, you can only take VIJOICE tablets.
- Do not use multiple packets of 50 mg oral granules or part of a packet to prepare a dose. Do not combine VIJOICE tablets with VIJOICE oral granules to prepare your prescribed dose.
- If you miss a dose of VIJOICE, you may take it with food up to 9 hours after the time you usually take it. If it has been more than 9 hours after you usually take your dose, skip the dose for that day. The next day, take the dose at your usual time. Do not take 2 doses to make up for a missed dose.
- If you vomit after taking a dose of VIJOICE, do not take another dose on that day. Take your next dose at your usual time.
- If you take too much VIJOICE, call your healthcare provider or go to the nearest hospital emergency room right away.
- VIJOICE tablets can be swallowed whole, or prepared as a suspension to take or give by mouth or through a feeding tube.
- If you are taking or giving VIJOICE tablets whole:
- Swallow VIJOICE tablets whole and take with food. Do not chew, divide, or crush the tablets.
- Do not take VIJOICE tablets that are broken, cracked, or that look damaged.
- If you cannot swallow tablets whole or need to use a feeding tube, you will need to mix VIJOICE tablets with water before taking or giving the dose.
- VIJOICE oral granules can be placed directly onto the tongue and swallowed with water, or mixed with a beverage or soft food to take or give by mouth, or mixed with water to give through a feeding tube.
What are the possible side effects of VIJOICE?
VIJOICE may cause serious side effects, including:- Severe allergic reactions. Tell your healthcare provider or get medical help right away if you have trouble breathing, swelling of the face or throat, flushing, rash, fever, or fast heart rate during treatment with VIJOICE.
- Severe skin reactions. Tell your healthcare provider or get medical help right away if you get severe rash or rash that keeps getting worse, reddened skin, flu-like symptoms, blistering of the lips, eyes or mouth, blisters on the skin or skin peeling, with or without fever.
- High blood sugar levels (hyperglycemia). Hyperglycemia is common with VIJOICE and may be severe. Your healthcare provider will monitor your sugar levels before you start and during treatment with VIJOICE. Your healthcare provider may monitor your sugar levels more often if you have a history of diabetes. Tell your healthcare provider right away if you develop symptoms of hyperglycemia, including:
◦ excessive thirst
◦ dry mouth
◦ more frequent urination than usual or a higher amount of urine than normal
◦ increased appetite with weight loss◦ confusion
◦ nausea
◦ vomiting
◦ fruity odor on breath
◦ difficulty breathing
◦ dry or flushed skin- Lung problems (pneumonitis). Tell your healthcare provider right away if you develop new or worsening symptoms of lung problems, including:
◦ shortness of breath or trouble breathing
◦ cough◦ chest pain - Diarrhea or colitis (inflammation of your intestines). Diarrhea is common with VIJOICE and may be severe. Severe diarrhea can lead to the loss of too much body water (dehydration) and kidney injury. Tell your healthcare provider right away if you develop diarrhea, stomach-area (abdominal) pain, or see mucus or blood in your stool during treatment with VIJOICE. Your healthcare provider may tell you to drink more fluids or take medicines to treat diarrhea or colitis.
Your healthcare provider may tell you to decrease your dose, temporarily stop your treatment, or completely stop your treatment with VIJOICE if you get certain serious side effects.
The most common side effects of VIJOICE include:diarrhea mouth sores (stomatitis) high blood sugar VIJOICE may cause hair loss (alopecia). Talk to a skin specialist (dermatologist) if this is a concern for you.
VIJOICE may affect fertility in males and in females who are able to become pregnant. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of VIJOICE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VIJOICE? - Store VIJOICE at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of VIJOICE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VIJOICE for a condition for which it was not prescribed. Do not give VIJOICE to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about VIJOICE that is written for health professionals.What are the ingredients in VIJOICE?
Active ingredient: alpelisib
Inactive ingredients for VIJOICE tablets: hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate. The film-coating contains hypromellose, iron oxide red (applicable only to 50 mg and 200 mg strengths), iron oxide yellow, macrogol/polyethylene glycol (PEG) 4000, talc, and titanium dioxide.
Inactive ingredients for VIJOICE oral granules: hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate.
Distributed by: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey 07936
© Novartis
For more information, go to www.VIJOICE.com or call 1-888-VIJOICE (1-888-845-6423).T2025-39
-
INSTRUCTIONS FOR USE
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 7/2025 INSTRUCTIONS FOR USE
VIJOICE® [vi’ joiz]
(alpelisib)
tabletsThis Instructions for Use contains information on how to prepare and take or give a dose of VIJOICE tablets. Read this Instructions for Use carefully before taking or giving VIJOICE tablets. Talk to your healthcare provider or pharmacist if you have any questions. You can also call Novartis Pharmaceuticals Corporation at 1-888-845-6423 for more information. Important Information You Need to Know Before Taking or Giving VIJOICE - Take or give VIJOICE exactly as your healthcare provider tells you.
- Do not change your dose or stop taking VIJOICE unless your healthcare provider tells you.
- Take VIJOICE 1 time each day, at about the same time each day.
- Take VIJOICE with food.
- Do not combine VIJOICE tablets with VIJOICE oral granules to prepare your prescribed dose.
- If you miss a dose of VIJOICE, you may take it with food up to 9 hours after the time you usually take it. If it has been more than 9 hours after you usually take your dose, skip the dose for that day. The next day, take the dose at your usual time. Do not take 2 doses to make up for a missed dose.
- If you vomit after taking a dose of VIJOICE, do not take another dose on that day. Take your next dose at your usual time.
- If you take too much VIJOICE, call your healthcare provider or go to the nearest hospital emergency room right away.
Storing VIJOICE tablets - Store VIJOICE tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep VIJOICE and all medicines out of the reach of children.
Preparing and taking or giving VIJOICE tablets
You can take or give VIJOICE tablets whole by mouth, or mixed with water (suspension) by mouth or through a feeding tube as described in the sections below:
Taking or giving VIJOICE tablets whole by mouth
Swallow VIJOICE tablets whole and take with food.
- Do not chew, divide, or crush the tablets.
- Do not take VIJOICE tablets that are broken, cracked, or that look damaged.
Taking or giving VIJOICE tablets mixed with water (suspension) by mouth
If you cannot swallow tablets whole, VIJOICE tablets can be prepared as a suspension and taken or given by mouth.
Gather the following supplies (see Figure A):- the number of VIJOICE tablet(s) needed for your prescribed dose
- water
- a clean empty cup
- a spoon
Step 2. Place VIJOICE tablets into a cup that contains 2 to 4 ounces of water. Use water only.
Step 3. Let the tablets stand in water for about 5 minutes.
Step 4. Crush the tablets with a spoon and stir to disperse the tablets in water (suspension). The suspension will be cloudy and you may see tablet particles (see Figure B).
Step 5. Drink all of the suspension right away. If you cannot take or give it right away, take or give the suspension within 60 minutes of preparing the dose. Stir the suspension again with the same spoon before drinking it.
Step 6. Add about 1 to 2 ounces of water to the same cup and stir with the same spoon. Then drink all of the suspension to make sure the entire dose is taken.
Step 7. Repeat Step 6 until there are no tablet particles remaining in the cup.
Step 8. Wash your hands and all supplies used to take or give VIJOICE tablets.
Throw away any VIJOICE suspension that is not taken within 60 minutes after it is prepared.
Figure A 
Figure B
Giving VIJOICE tablets mixed with water (suspension) through a feeding tube
VIJOICE tablets may be given through a feeding tube, according to the manufacturer’s instructions and as directed by your healthcare provider. Only use silicone or polyurethane nasogastric tubes with a French size 5 to 12, or silicone gastric tubes with a French size 12 to 24.
Gather the following supplies (see Figure C):- the number of VIJOICE tablet(s) needed for your prescribed dose
- water
- a clean empty cup
- a spoon
- an enteral syringe
Figure C 
Step 1. Wash and dry your hands.
Step 2. Place VIJOICE tablets into a cup that contains 1 to 2 ounces (about 30 to 60 mL) of water. Use water only.
Step 3. Let the tablets stand in water for about 5 minutes.
Step 4. Crush the tablets with a spoon and stir to disperse the tablets in water (suspension). The suspension will be cloudy and you may see tablet particles (see Figure B).
Step 5. Draw up all of the suspension from the cup into an enteral syringe (see Figure D) and give it through the feeding tube right away. If you cannot give it right away, give the suspension within 60 minutes of preparing the dose. Stir the suspension again with the same spoon before drawing it up.
Step 6. Add about 1 to 2 ounces (about 30 to 60 mL) of water to the same cup and stir with the same spoon.
Step 7. Draw up all of the suspension into the same enteral syringe and give it through the feeding tube.
Step 8. Repeat Steps 6 and 7 if there are tablet particles remaining in the enteral syringe or in the cup.
Step 9. Wash your hands and all supplies used to give VIJOICE tablets. Follow the manufacturer’s instructions to clean your enteral syringe.
Throw away any VIJOICE suspension that is not taken within 60 minutes after it is prepared.Figure D 
Distributed by:
Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936
© NovartisT2025-40
-
INSTRUCTIONS FOR USE
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 7/2025 INSTRUCTIONS FOR USE
VIJOICE® [vi’ joiz]
(alpelisib)
oral granulesThis Instructions for Use contains information on how to prepare and take or give a dose of VIJOICE oral granules. Read this Instructions for Use carefully before taking or giving VIJOICE oral granules. Talk to your healthcare provider or pharmacist if you have any questions. You can also call Novartis Pharmaceuticals Corporation at 1-888-845-6423 for more information. Important Information You Need to Know Before Taking or Giving VIJOICE - Take or give VIJOICE exactly as your healthcare provider tells you.
- Do not change your dose or stop taking VIJOICE unless your healthcare provider tells you.
- Take VIJOICE 1 time each day, at about the same time each day.
- Take VIJOICE with food.
- Each VIJOICE oral granules packet is for single use only.
- Do not use the packet if the packet seal is broken.
- Do not use multiple packets of 50 mg oral granules or part of a packet to prepare a dose.
- Do not combine VIJOICE tablets with VIJOICE oral granules to prepare your prescribed dose.
- If you miss a dose of VIJOICE, you may take it with food up to 9 hours after the time you usually take it. If it has been more than 9 hours after you usually take your dose, skip the dose for that day. The next day, take the dose at your usual time. Do not take 2 doses to make up for a missed dose.
- If you vomit after taking a dose of VIJOICE, do not take another dose on that day. Take your next dose at your usual time.
- If you take too much VIJOICE, call your healthcare provider or go to the nearest hospital emergency room right away.
Storing VIJOICE oral granules - Store VIJOICE oral granules at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep VIJOICE and all medicines out of the reach of children.
Preparing and taking or giving VIJOICE oral granules You can take or give VIJOICE oral granules directly onto the tongue swallowed with water, or mixed with a beverage or soft food by mouth, or mixed with water through a feeding tube as described in the sections below: Taking or giving VIJOICE oral granules directly onto the tongue
Gather the following supplies (see Figure A):- 1 VIJOICE oral granules packet
- scissors
- water
Step 2. Hold the packet of oral granules with the cut line on top.
Step 3. Shake the packet gently to ensure the VIJOICE oral granules are in the lower part of the packet.
Step 4. Using scissors, open the packet along the cut line.
Step 5. Pour all the oral granules from 1 VIJOICE oral granules packet directly onto the tongue by tapping the side and top of the packet, and swallow it with about 2 to 4 ounces of water (see Figure B).
Step 6. If there is any medicine remaining in the mouth, rinse the mouth with additional water and swallow to make sure the entire dose is taken.
Step 7. Wash your hands and all supplies used to take or give VIJOICE oral granules.Figure A  Figure B
Figure B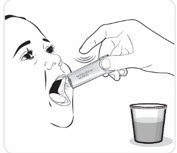
Taking or giving VIJOICE oral granules mixed with a beverage or soft food by mouth
Gather the following supplies (see Figure C):- 1 VIJOICE oral granules packet
- scissors
- the beverage or soft food of your choice among water, milk, apple juice, applesauce or yogurt. See Step 6.
- a clean empty cup
- a spoon
Figure C 
Step 1. Wash and dry your hands.
Step 2. Hold the packet of oral granules with the cut line on top.
Step 3. Shake the packet gently to ensure the VIJOICE oral granules are in the lower part of the packet.
Step 4. Using scissors, open the packet along the cut line.
Step 5. Pour the oral granules from 1 VIJOICE oral granules packet into a cup by tapping the side and top of the packet (see Figure D).
Step 6. Add 1 to 3 teaspoons of a beverage (water, milk, or apple juice) or soft food (applesauce or yogurt), stir with a spoon and take or give the mixture right away. If you do not take or give it right away, take or give the mixture within 60 minutes of preparing the dose. Stir the mixture with the same spoon before taking or giving it.
Step 7. Rinse the cup with up to 2 ounces of water, milk, or apple juice. Drink the rinse right away to make sure the entire dose is taken. Repeat this step until there are no oral granules remaining in the cup.
Step 8. Wash your hands and all supplies used to take or give VIJOICE oral granules.
Throw away any VIJOICE oral granules mixture that is not taken within 60 minutes after it is prepared.Figure D 
Giving VIJOICE oral granules mixed with water (suspension) through a feeding tube
VIJOICE oral granules may be given through a feeding tube, according to the manufacturer’s instructions and as directed by your healthcare provider. Only use silicone or polyurethane nasogastric tubes with a French size 8 to 12, or silicone gastric tubes with a French size 12 to 24.
Gather the following supplies (see Figure E):- 1 VIJOICE oral granules packet
- water
- a clean empty cup
- a spoon
- an enteral syringe
Figure E 
Step 1. Wash and dry your hands.
Step 2. Pour the oral granules from 1 VIJOICE oral granules packet into a cup by tapping the side and top of the packet (see Figure D).
Step 3. Add 4 teaspoons (about 20 mL) of water to the same cup and stir gently with a spoon to disperse the oral granules in the water (suspension). Use water only.
Step 4. Draw up all of the suspension from the cup into an enteral syringe (see Figure F) and give it through the feeding tube right away. If you cannot give it right away, give the suspension within 60 minutes of preparing the dose. Stir the suspension again with the same spoon before drawing it up.
Step 5. Add 4 teaspoons (about 20 mL) of water to the same cup and stir with the same spoon.
Step 6. Draw up all of the suspension from the cup into the same enteral syringe and give it through the feeding tube.
Step 7. Repeat Steps 5 and 6 until there are no oral granules remaining in the cup or in the syringe.
Step 8. Wash your hands and all supplies used give VIJOICE oral granules. Follow the manufacturer’s instructions to clean your enteral syringe.
Throw away any VIJOICE suspension that is not taken within 60 minutes after it is prepared.Figure F 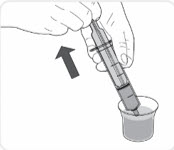
Distributed by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936
© NovartisT2025-41
-
PRINCIPAL DISPLAY PANEL
Vijoice®
(alpelisib) tabletsNDC: 0078-1021-84
50 mg daily dose
Take one 50 mg tablet once daily
Rx only
Recommended Dosage: Take one 50 mg tablet once daily with food.
Swallow tablets whole. DO NOT chew, crush, or split tablets.Directions: See enclosed Instructions for Use for administration
instructions, including options for patients who are unable to swallow
whole tablets.28-Day Supply
Contains: One blister pack containing 28 tablets
NOVARTIS
-
PRINCIPAL DISPLAY PANEL
Vijoice®
(alpelisib) tabletsNDC: 0078-1028-84
125 mg daily dose
Take one 125 mg tablet once daily
Rx only
Recommended Dosage: Take one 125 mg tablet once daily with food.
Swallow tablets whole. DO NOT chew, crush, or split tablets.Directions: See enclosed Instructions for Use for administration
instructions, including options for patients who are unable to swallow
whole tablets.28-Day Supply
Contains: One blister pack containing 28 tablets
NOVARTIS
-
PRINCIPAL DISPLAY PANEL
Vijoice®
(alpelisib) tabletsNDC: 0078-1035-02
250 mg daily dose
Take one 200 mg tablet and one 50 mg tablet once daily
Rx only
Recommended Dosage: Take one 200 mg tablet and one 50 mg
tablet once daily with food. Swallow tablets whole. DO NOT chew,
crush, or split tablets.Directions: See enclosed Instructions for Use for administration
instructions, including options for patients who are unable to
swallow whole tablets.28-Day Supply (56 Tablets)
Contains: Two 14-day blister packs each containing 28 tablets:
200 mg
14 tablets per pack50 mg
14 tablets per packNOVARTIS
-
PRINCIPAL DISPLAY PANEL
Rx only
NDC: 0078-1175-51
Vijoice®
(alpelisib) oral granules50 mg daily dose
Take one 50 mg packet once daily
Directions: See enclosed Instructions For
Use for administration instructions.28 Packets
NOVARTIS

-
INGREDIENTS AND APPEARANCE
VIJOICE
alpelisib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-1021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPELISIB (UNII: 08W5N2C97Q) (ALPELISIB - UNII:08W5N2C97Q) ALPELISIB 50 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (light) Score no score Shape ROUND (round and curved with beveled edges) Size 7mm Flavor Imprint Code C7;NVR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-1021-84 1 in 1 CARTON 04/05/2022 1 NDC: 0078-1021-51 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0078-1021-91 1 in 1 CARTON 04/05/2022 2 NDC: 0078-1021-90 28 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215039 04/05/2022 VIJOICE
alpelisib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-1028 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPELISIB (UNII: 08W5N2C97Q) (ALPELISIB - UNII:08W5N2C97Q) ALPELISIB 125 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (dark) Score no score Shape OVAL (ovaloid and curved with beveled edges) Size 13mm Flavor Imprint Code Y7;NVR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-1028-84 1 in 1 CARTON 04/05/2022 1 NDC: 0078-1028-51 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0078-1028-91 1 in 1 CARTON 04/05/2022 2 NDC: 0078-1028-90 28 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215039 04/05/2022 VIJOICE
alpelisib kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-1035 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-1035-02 2 in 1 CARTON 04/05/2022 1 NDC: 0078-1035-61 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0078-1035-92 2 in 1 CARTON 04/05/2022 2 NDC: 0078-1035-94 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 14 Part 2 14 Part 1 of 2 VIJOICE
alpelisib tabletProduct Information Item Code (Source) NDC: 0078-1133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPELISIB (UNII: 08W5N2C97Q) (ALPELISIB - UNII:08W5N2C97Q) ALPELISIB 200 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color YELLOW (pale) Score no score Shape OVAL (ovaloid and curved with beveled edges) Size 16mm Flavor Imprint Code CL7;NVR Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215039 04/05/2022 Part 2 of 2 VIJOICE
alpelisib tabletProduct Information Item Code (Source) NDC: 0078-1140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPELISIB (UNII: 08W5N2C97Q) (ALPELISIB - UNII:08W5N2C97Q) ALPELISIB 50 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (light) Score no score Shape ROUND (round and curved with beveled edges) Size 7mm Flavor Imprint Code C7;NVR Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215039 04/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215039 04/05/2022 VIJOICE
alpelisib granuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-1175 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPELISIB (UNII: 08W5N2C97Q) (ALPELISIB - UNII:08W5N2C97Q) ALPELISIB 50 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-1175-51 28 in 1 CARTON 04/24/2024 1 NDC: 0078-1175-19 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218466 04/24/2024 Labeler - Novartis Pharmaceuticals Corporation (002147023)
Trademark Results [VIJOICE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIJOICE 79352744 not registered Live/Pending |
NOVARTIS AG 2022-09-13 |
 VIJOICE 79284023 not registered Live/Pending |
NOVARTIS AG 2020-03-10 |
 VIJOICE 79281646 not registered Live/Pending |
NOVARTIS AG 2020-01-28 |
 VIJOICE 79249982 not registered Live/Pending |
NOVARTIS AG 2018-12-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.