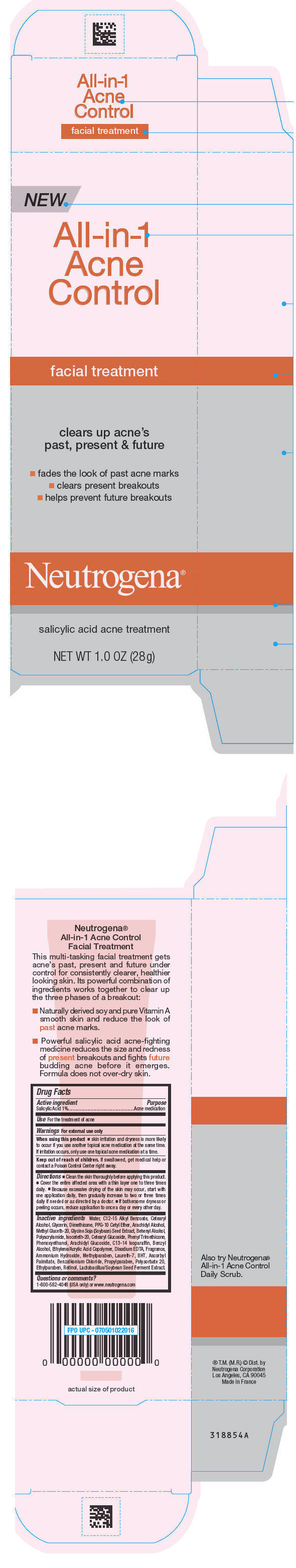
Neutrogena All-in-1 Acne Control Facial Treatment
Neutrogena All-in-1 Acne Control by
Drug Labeling and Warnings
Neutrogena All-in-1 Acne Control by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NEUTROGENA ALL-IN-1 ACNE CONTROL FACIAL TREATMENT- salicylic acid cream
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Neutrogena All-in-1 Acne Control Facial Treatment
Warnings
For external use only
Directions
- Clean the skin thoroughly before applying this product
- Cover the entire affected area with a thin layer one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
Water
C12-15 Alkyl Benzoate
Cetearyl Alcohol
Glycerin
Dimethicone
PPG-10 Cetyl Ether
Arachidyl Alcohol
Methyl Gluceth-20
Glycine Soja (Soybean) Seed Extract
Behenyl Alcohol
Polyacrylamide
Isoceteth-20
Cetearyl Glucoside
Phenyl Trimethicone
Phenoxyethanol
Arachidyl Glucoside
C13-14 Isoparaffin
Benzyl Alcohol
Ethylene/Acrylic Acid Copolymer
Disodium EDTA
Fragrance
Ammonium Hydroxide
Methylparaben
Laureth-7
BHT
Ascorbyl Palmitate
Benzalkonium Chloride
Propylparaben
Polysorbate 20
Ethylparaben
Retinol
Lactobacillus/Soybean Seed Ferment Extract
| NEUTROGENA ALL-IN-1 ACNE CONTROL
FACIAL TREATMENT
salicylic acid cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |