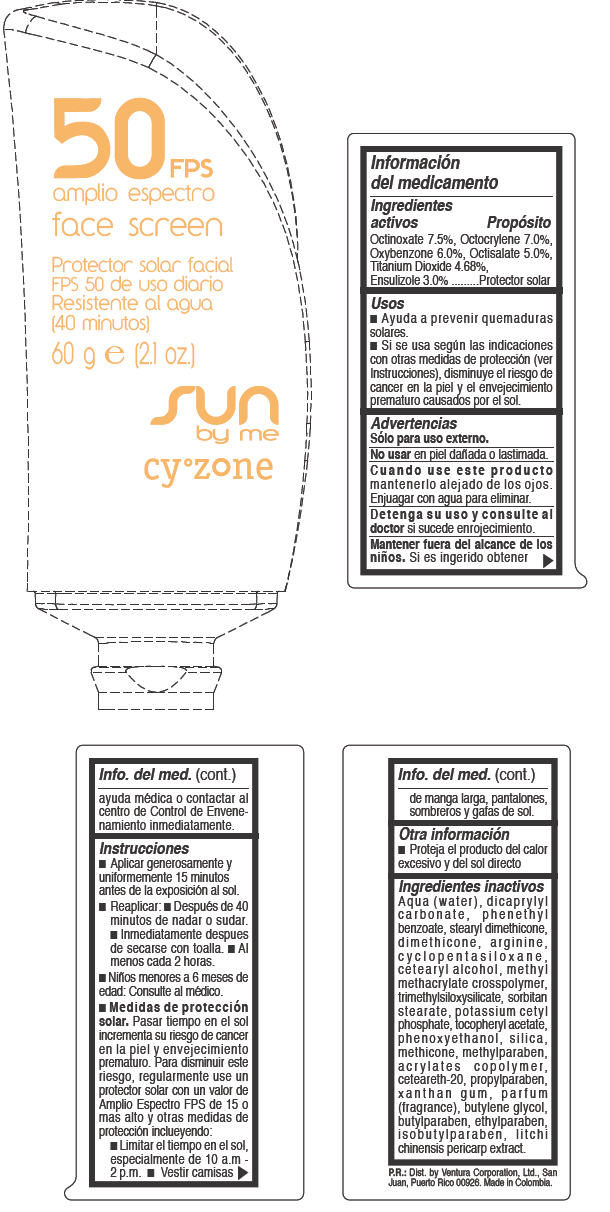
CYZONE SUN BY ME FACE SCREEN FPS 50 AMPLIO ESPECTRO- octinoxate, octocrylene, oxybenzone, octisalate, titanium dioxide, and ensulizole cream
CYZONE by
Drug Labeling and Warnings
CYZONE by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD., Bel Star S.A. (Colombia), Yobel Supply Chain Management S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Ingredientes activos
- Propósito
-
Usos
- Ayuda a prevenir quemaduras solares.
- Si se usa según las indicaciones con otras medidas de protección (ver Instrucciones), disminuye el riesgo de cancer en la piel y el envejecimiento prematuro causados por el sol.
- Advertencias
-
Instrucciones
- Aplicar generosamente y uniformemente 15 minutos antes de la exposición al sol.
- Reaplicar:
- Después de 40 minutos de nadar o sudar.
- Inmediatamente despues de secarse con toalla
- Al menos cada 2 horas.
- Niños menores a 6 meses de edad: Consulte al médico
-
Medidas de protección solar. Pasar tiempo en el sol incrementa su riesgo de cancer en la piel y envejecimiento prematuro. Para disminuir este riesgo, regularmente use un protector solar con un valor de Amplio Espectro FPS de 15 o mas alto y otras medidas de protección inclueyendo:
- Limitar el tiempo en el sol, especialmente de 10 a.m - 2 p.m.
- Vestir camisas de manga larga, pantalones, sombreros y gafas de sol.
- Otra información
-
Ingredientes inactivos
AQUA (WATER), DICAPRYLYL CARBONATE, PHENETHYL BENZOATE, STEARYL DIMETHICONE, DIMETHICONE, ARGININE, CYCLOPENTASILOXANE, CETEARYL ALCOHOL, METHYL METHACRYLATE CROSSPOLYMER, TRIMETHYLSILOXYSILICATE, SORBITAN STEARATE, POTASSIUM CETYL PHOSPHATE, TOCOPHERYL ACETATE, PHENOXYETHANOL, SILICA, METHICONE, METHYLPARABEN, ACRYLATES COPOLYMER, CETEARETH-20, PROPYLPARABEN, XANTHAN GUM, PARFUM (FRAGRANCE), BUTYLENE GLYCOL, BUTYLPARABEN, ETHYLPARABEN, ISOBUTYLPARABEN, LITCHI CHINENSIS PERICARP EXTRACT.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 g Tube Label
-
INGREDIENTS AND APPEARANCE
CYZONE SUN BY ME FACE SCREEN FPS 50 AMPLIO ESPECTRO
octinoxate, octocrylene, oxybenzone, octisalate, titanium dioxide, and ensulizole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-435 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.07 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.06 g in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.05 g in 1 g Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.468 g in 1 g Ensulizole (UNII: 9YQ9DI1W42) (Ensulizole - UNII:9YQ9DI1W42) Ensulizole 0.03 g in 1 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dicaprylyl carbonate (UNII: 609A3V1SUA) phenethyl benzoate (UNII: 0C143929GK) dimethicone (UNII: 92RU3N3Y1O) arginine (UNII: 94ZLA3W45F) cyclomethicone 5 (UNII: 0THT5PCI0R) cetostearyl alcohol (UNII: 2DMT128M1S) sorbitan monostearate (UNII: NVZ4I0H58X) potassium cetyl phosphate (UNII: 03KCY6P7UT) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) phenoxyethanol (UNII: HIE492ZZ3T) silicon dioxide (UNII: ETJ7Z6XBU4) methylparaben (UNII: A2I8C7HI9T) polyoxyl 20 cetostearyl ether (UNII: YRC528SWUY) propylparaben (UNII: Z8IX2SC1OH) xanthan gum (UNII: TTV12P4NEE) butylene glycol (UNII: 3XUS85K0RA) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) isobutylparaben (UNII: 0QQJ25X58G) litchi fruit (UNII: Y5P61KP51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-435-01 60 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/14/2012 Labeler - Ventura Corporation LTD. (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-435) Establishment Name Address ID/FEI Business Operations Yobel Supply Chain Management S.A. 934116930 MANUFACTURE(13537-435)
Trademark Results [CYZONE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYZONE 90644534 not registered Live/Pending |
FUJIAN QUANZHOU CYNICE IMPORT AND EXPORT TRADING CO., LTD 2021-04-14 |
 CYZONE 90298521 not registered Live/Pending |
EBEL INTERNATIONAL LIMITED 2020-11-04 |
 CYZONE 72169340 0764507 Dead/Expired |
AMERICAN CYANAMID COMPANY 1963-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.