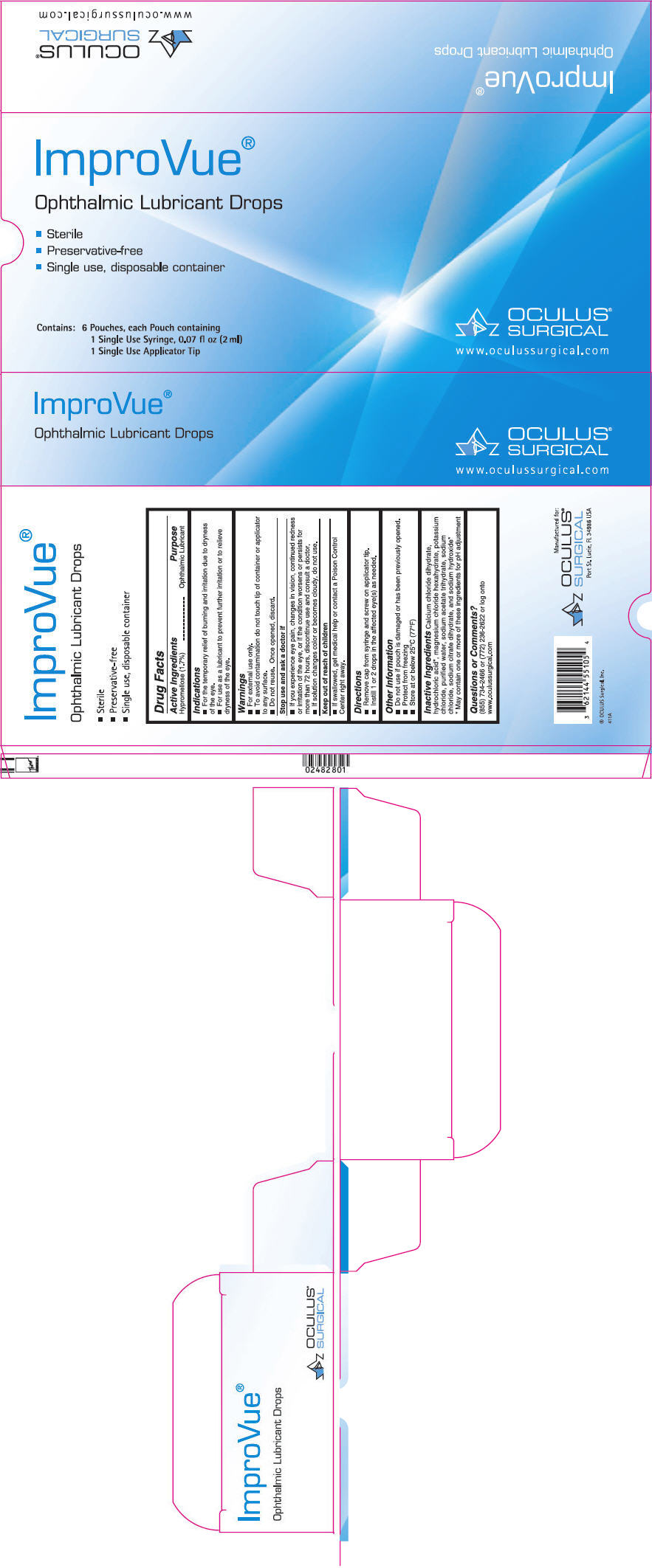
IMPROVUE LUBRICANT (hypromellose 2208- 15000 mpa.s solution/ drops
ImproVue by
Drug Labeling and Warnings
ImproVue by is a Otc medication manufactured, distributed, or labeled by Oculus Surgical, Inc., OASIS Medical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Indications
-
Warnings
- For external use only.
- To avoid contamination do not touch tip of container or applicator to any surface.
- Do not reuse. Once opened, discard.
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL - 6 Pouch Carton
-
INGREDIENTS AND APPEARANCE
IMPROVUE LUBRICANT
hypromellose 2208 (15000 mpa.s) solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62144-5510 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) (HYPROMELLOSE 2208 (15000 MPA.S) - UNII:Z78RG6M2N2) HYPROMELLOSE 2208 (15000 MPA.S) 17 mg in 1 mL Inactive Ingredients Ingredient Name Strength Calcium Chloride (UNII: M4I0D6VV5M) Hydrochloric Acid (UNII: QTT17582CB) Magnesium Chloride (UNII: 02F3473H9O) Potassium Chloride (UNII: 660YQ98I10) Water (UNII: 059QF0KO0R) Sodium Acetate (UNII: 4550K0SC9B) Sodium Chloride (UNII: 451W47IQ8X) Trisodium Citrate Dihydrate (UNII: B22547B95K) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62144-5510-5 6 in 1 CARTON 06/25/2014 1 1 in 1 POUCH 1 2 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part349 06/25/2014 Labeler - Oculus Surgical, Inc. (015409582) Establishment Name Address ID/FEI Business Operations OASIS Medical, Inc. 194121018 MANUFACTURE(62144-5510) Establishment Name Address ID/FEI Business Operations OASIS Medical, Inc. 024362989 PACK(62144-5510)
Trademark Results [ImproVue]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMPROVUE 85875325 4619436 Live/Registered |
Oculus Optikgeraete GmbH 2013-03-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.