IXCHIQ- chikungunya vaccine, live-attenuated injection, powder, lyophilized, for solution
IXCHIQ by
Drug Labeling and Warnings
IXCHIQ by is a Other medication manufactured, distributed, or labeled by Valneva Scotland Ltd., Valneva Austria GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
IXCHIQ
These highlights do not include all the information needed to use IXCHIQ. See full prescribing information for IXCHIQ.
Initial U.S. Approval: 2023INDICATIONS AND USAGE
IXCHIQ is a vaccine indicated for the prevention of disease caused by chikungunya virus (CHIKV) in individuals 18 years of age and older who are at increased risk of exposure to CHIKV. (1)
This indication is approved under accelerated approval based on anti-CHIKV neutralizing antibody titers. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory studies.
DOSAGE AND ADMINISTRATION
For intramuscular use only.
Administer IXCHIQ as a single approximately 0.5 mL dose. (2.3)
DOSAGE FORMS AND STRENGTHS
IXCHIQ is a solution for injection. After reconstitution, a single dose is approximately 0.5 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- IXCHIQ may cause severe or prolonged chikungunya-like adverse reactions. (5.2)
- Vertical transmission of wild-type CHIKV from pregnant individuals with viremia at delivery is common and can cause potentially fatal CHIKV disease in neonates. Vaccine viremia occurs in the first week following administration of IXCHIQ, with resolution of viremia by 14 days after vaccination. It is not known if the vaccine virus can be vertically transmitted and cause fetal or neonatal adverse reactions. (5.3)
- Syncope (fainting) may occur in association with administration of injectable vaccines, including IXCHIQ. Procedures should be in place to avoid injury from fainting. (5.4)
ADVERSE REACTIONS
In clinical studies, the most common solicited injection site reaction (>10%) was tenderness (10.6%). The most common solicited systemic adverse reactions (>10%) were headache (31.6%), fatigue (28.5%), myalgia (23.9%), arthralgia (17.2%), fever (13.5%) and nausea (11.2%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Valneva USA Inc. at 1-844-349-4276 or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
A decision to administer IXCHIQ during pregnancy should take into consideration the individual’s risk of wild-type CHIKV infection, gestational age, and risks to the fetus or neonate from vertical transmission of wild-type CHIKV. (8.1)
See 17 for FDA-approved patient labeling.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Schedule
2.2 Preparation for Administration
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Immunocompromised Individuals
4.2 Severe Allergic Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Risk of Severe or Prolonged Chikungunya-like Adverse Reactions
5.3 Potential for Vertical Transmission of Vaccine Virus and Fetal/Neonatal Adverse Reactions
5.4 Syncope
5.5 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
Text from principal display panel
Image of carton
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
IXCHIQ is a vaccine indicated for the prevention of disease caused by chikungunya virus (CHIKV) in individuals 18 years of age and older who are at increased risk of exposure to CHIKV.
This indication is approved under accelerated approval based on anti-CHIKV neutralizing antibody levels [See Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory studies.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Recommended Dose and Schedule
After reconstitution, a single dose of IXCHIQ is approximately 0.5 mL.
Administer a single dose of IXCHIQ by intramuscular injection.
2.2 Preparation for Administration
Reconstitute the Lyophilized Antigen Component, Live (a white to slightly yellowish powder) only with the accompanying Sterile Water Diluent Component to form IXCHIQ. The reconstituted vaccine is a clear, colorless to slightly yellowish liquid solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, do not administer the vaccine.
Figure 1 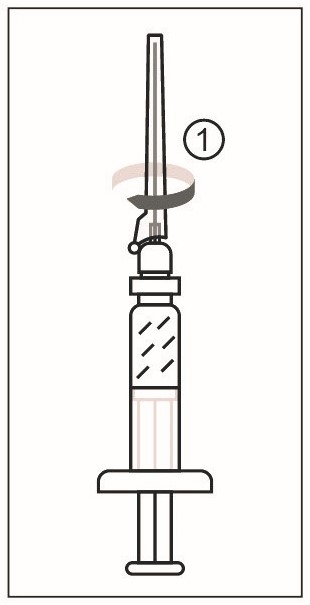
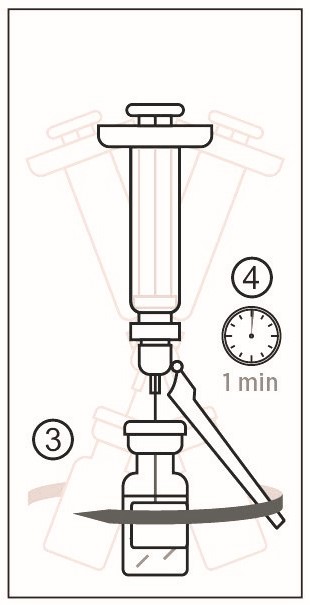
1) Remove the cap from the syringe of Sterile Water Diluent Component. Attach a needle to the Luer lock of the syringe. Figure 2 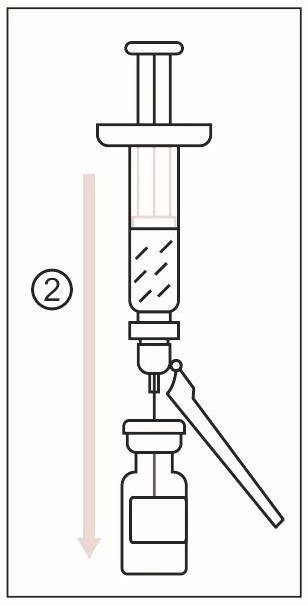
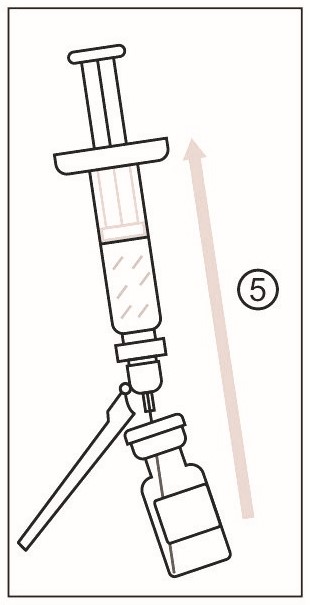
2) Cleanse the stopper of the vial of Lyophilized Antigen Component, Live. Slowly transfer the entire contents of the prefilled syringe of Sterile Water Diluent Component into the vial. - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Immunocompromised Individuals
Do not administer IXCHIQ to individuals who are immunodeficient or immunosuppressed due to disease or medical therapy (e.g., from hematologic and solid tumors, receipt of chemotherapy, congenital immunodeficiency, long-term immunosuppressive therapy or patients with HIV infection who are severely immunocompromised).
4.2 Severe Allergic Reactions
Do not administer IXCHIQ to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be available in the event an acute anaphylactic reaction occurs following administration of IXCHIQ.
5.2 Risk of Severe or Prolonged Chikungunya-like Adverse Reactions
Vaccination with IXCHIQ may cause severe or prolonged chikungunya-like adverse reactions. Severe chikungunya-like adverse reactions that prevented daily activity and/or required medical intervention occurred in 1.6% of 3,082 IXCHIQ recipients and no placebo recipients. Two IXCHIQ recipients required hospitalization for severe myalgia and for hypovolemic hyponatremia and atrial fibrillation, respectively. Fourteen IXCHIQ recipients had prolonged (duration at least 30 days) chikungunya-like adverse reactions [See Adverse Reactions (6.1)].
5.3 Potential for Vertical Transmission of Vaccine Virus and Fetal/Neonatal Adverse Reactions
Vertical transmission of wild-type CHIKV to neonates from pregnant individuals with viremia at delivery is common and can cause severe, potentially fatal CHIKV disease in neonates. Vertical transmission of wild-type CHIKV and fetal death attributable to CHIKV in the context of antepartum infection has been reported to occur infrequently [See Use in Specific Populations (8.1)].
Vaccine viremia occurs in the first week following administration of IXCHIQ, with resolution of viremia by 14 days after vaccination [See Clinical Pharmacology (12.2)]. It is not known if the vaccine virus can be transmitted from a pregnant individual to the fetus or neonate and cause fetal or neonatal adverse reactions.
Decisions to administer IXCHIQ during pregnancy should take into consideration the individual’s risk of exposure to wild-type CHIKV, gestational age, and risks to the fetus or neonate from vertical transmission of wild-type CHIKV.
Closely monitor neonates for 7 days after birth for potential disease due to vaccine virus if they are born within 14 days of their mother receiving IXCHIQ.
-
6 ADVERSE REACTIONS
In clinical studies, the most common solicited injection site reaction (>10%) was tenderness (10.6%). The most common solicited systemic adverse reactions (>10%) were headache (31.6%), fatigue (28.5%), myalgia (23.9%), arthralgia (17.2%), fever (13.5%) and nausea (11.2%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of IXCHIQ was evaluated in two clinical studies [Study 1 (NCT04546724), Study 2 (NCT04786444)], both conducted in North America, in which a total of 3,490 participants 18 years of age and older received a dose of IXCHIQ.), Study 2 (NCT04786444)], both conducted in North America, in which a total of 3,490 participants 18 years of age and older received a dose of IXCHIQ.
Study 1 was a randomized, placebo-controlled, double-blinded study, in which participants were vaccinated with a single dose of IXCHIQ (n=3,082) or placebo (Phosphate Buffered Saline) (n=1,033). Study 2 was a non-placebo-controlled study where 408 participants were vaccinated with a single dose of IXCHIQ. Among the overall 4,523 participants enrolled in those studies, 54.7% were female; 80.1% were White, 14.0% Black or African American, 1.9% Asian, 0.8% American Indian or Alaska Native, 0.4% Native Hawaiian or Pacific Islander, 2.7% Other racial groups; and 17.2% were Hispanic or Latino.
In Studies 1 and 2, solicited adverse reactions, including injection site and systemic adverse reactions, were collected via electronic diary in the first 10 days post-vaccination. An electronic memory aid was distributed to each participant for the collection of safety information outside the first ten post-vaccination days until the next visit. Unsolicited adverse events, chikungunya-like adverse reactions, and serious adverse events were monitored through 6 months post-vaccination. In Study 1, hematology parameters were assessed at enrollment, 7, 28, 84 and 180 days post-vaccination in a subset of participants who were evaluated for seroresponse to vaccination (immunogenicity subset). [See Clinical Studies (14)].
Solicited Adverse Reactions
In Study 1, the median age of participants was 45 years (range 18 through 94 years); 54.7% were female and 45.3% male; 80.4% were White, 13.9% Black or African American, 1.7% Asian, 0.8% American Indian or Alaska Native, 0.4% Native Hawaiian or Pacific Islander, 2.8% Other racial groups; and 17.5% were Hispanic or Latino. IXCHIQ and placebo groups were similar with regard to demographics.
The percentage of participants in Study 1 reporting solicited local and systemic adverse reactions is shown in Table 1. The median day of onset was Day 2 for local reactions (Day 1 was the day of vaccination) and Day 5 for systemic reactions. Local and systemic adverse reactions resolved with a median duration of 2 days.
Table 1. Percentage of Participants with Solicited Local and Systemic Adverse Reactions Within 10 Days After Vaccination (Study 1§) Category IXCHIQ
(N=3,082)
%Placebo
(N=1,033)
%Solicited Injection Site Adverse Reactiona Tenderness (any)b 10.6 8.1 Tenderness (severe) 0 0 Pain (any)c 6.2 3.7 Pain (severe) 0.03 0 Erythema/Redness (≥ 2.5 cm)d 1.5 1.5 Induration (≥ 2.5 cm)d 1.4 0.8 Swelling (≥ 2.5 cm)d 0.7 0.8 Solicited Systemic Adverse Reactiona Headache (any)a 31.6 14.7 Headache (severe)e 0.1 0.1 Fatigue (any)a 28.5 12.7 Fatigue (severe)e 0.2 0 Myalgia/Muscle Pain (any)a 23.9 7.4 Myalgia/Muscle Pain (severe)e 0.3 0 Arthralgia/Joint Pain (any)a 17.2 4.9 Arthralgia/Joint Pain (severe)e 0.3 0 Fever (any)f 13.5 0.9 Fever (severe or worse)g 1.4 0 Nausea (any)a 11.2 5.6 Nausea (severe)e 0 0.1 Rash (any)a 2.3 0.5 Rash (severe)h 0 0 Vomiting (any)a 1.9 1.0 Vomiting (severe)e 0 0.1 §NCT04546724
N=Number of participants
a Severity=mild, moderate, severe intensity.
b defined as mild (mild discomfort to touch), moderate (discomfort with movement), severe (significant discomfort at rest). Any potentially life threatening event (emergency room visit or hospitalization) was to be reported as severe.
c defined as mild (does not interfere with activity), moderate (repeated use of non-narcotic pain reliever > 24 hours or interferes with activity), severe (any use of narcotic pain reliever or prevents daily activity). Any potentially life threatening event (emergency room visit or hospitalization) was to be reported as severe.
d No participants had erythema, induration or swelling >10 cm
e Severe=Prevents daily activity (for fatigue, myalgia, and arthralgia); Prevents daily activity and requires medical intervention (for headache, nausea, vomiting)
f defined as temperature ≥38.0°C (100.4°F)
g defined as temperature ≥39.0°C (102.1°F)
h Severe=Macules/papules covering >30% body surface area with or without associated symptoms; limiting self-care activity of daily living.Hematology Parameters
In Study 1, hematology parameters were assessed at 7, 28, 84 and 180 days post-vaccination in the immunogenicity subset. Percentages of study participants with abnormally low leukocyte, neutrophil and lymphocyte counts are presented by maximum grade post-vaccination in Table 2. Abnormal leukocyte, neutrophil and lymphocyte counts were more frequently observed in IXCHIQ recipients than placebo recipients. Of 186 participants with abnormal cell counts at the 7-day post-vaccination assessment, 171 (171/186, 92%) had available hematology results at the 28-day post-vaccination assessment, of which 150 (150/171, 88%) were in the normal range.
Table 2. Abnormal Hematology Results by Maximum Grade Post-vaccination (Study 1§) Laboratory Parameter
GradeIXCHIQa (N=372)
n (%)Placeboa (N=125)
n (%)Leukocytes Grade 1 2,500 – 3,500 cell/mm3 99 (27.3) 7 (5.8) Grade 2 1,500 – 2,499 cell/mm3 16 (4.4) 0 Grade 3 1,000 – 1,499 cell/mm3 1 (0.3) 0 Grade 4 <1,000 cell/mm3 0 0 Neutrophils Grade 1 1,500 – 2,000 cell/mm3 100 (27.6) 14 (11.6) Grade 2 1,000 – 1,499 cell/mm3 41 (11.3) 1 (0.8) Grade 3 500 – 999 cell/mm3 11 (3.0) 0 Grade 4 < 500 cell/mm3 1 (0.3) 0 Lymphocytes Grade 1 750 – 1,000 cell/mm3 69 (19.1) 8 (6.6) Grade 2 500 – 749 cell/mm3 15 (4.1) 1 (0.8) Grade 3 250 – 499 cell/mm3 1 (0.3) 0 Grade 4 <250 cell/mm3 0 0 §NCT04546724, immunogenicity subset
a Individuals are included only once under the highest grade. Percentages are based on the number of individuals with at least one postbaseline result.Unsolicited Adverse Reactions
Unsolicited adverse events that occurred within 28 days following vaccination were reported in 21.8% of 3,082 participants who received IXCHIQ versus 13.3% of 1,033 participants who received placebo. Chills was reported by 1.8% of IXCHIQ recipients and 0.2% of placebo recipients; diarrhea was reported by 1.4% of IXCHIQ recipients and 0.4% of placebo recipients; back pain was reported by 1.1% of IXCHIQ recipients and 0.6% of placebo recipients, lymphadenopathy was reported by 0.9% of IXCHIQ recipients and 0% of placebo recipients. Chills, diarrhea, back pain, and lymphadenopathy are likely related to vaccination. There were no other notable patterns or numerical imbalances between treatment groups for specific categories of unsolicited adverse events that would suggest a causal relationship to IXCHIQ.
Serious Adverse Events
Among 3,490 IXCHIQ recipients, two (both from Study 1) reported serious adverse reactions. One IXCHIQ recipient reported myalgia and one reported atrial fibrillation and hypovolemic hyponatremia. These adverse reactions are further described under the subheading Chikungunya-Like Adverse Reactions.
Chikungunya-Like Adverse Reactions
Study 1, participants were monitored for a cluster of symptoms consistent with chikungunya. Chikungunya-like adverse reactions were defined as fever (38 °C / 100.4 °F) and one or more of any of the following: arthralgia or arthritis, myalgia, headache, back pain, rash, lymphadenopathy, or certain neurological, cardiac or ocular symptoms that occurred with an onset within 30 days after vaccination. Severe chikungunya-like adverse reactions were those that prevented daily activity and/or required medical intervention.
Among Study 1 participants, 361 (11.7%) in the IXCHIQ group (n= 3,082) reported chikungunya-like adverse reactions, including 48 participants (1.6%) who reported severe chikungunya-like adverse reactions. Six (0.6%) participants in the placebo group (n= 1,033) reported chikungunya-like adverse reactions, none of which were severe. The frequencies of chikungunya-like symptoms among the IXCHIQ recipients with chikungunya-like adverse reactions are presented in Table 3.
Table 3. Frequency of Chikungunya-Like Symptoms Among Participants With Chikungunya-Like Adverse Reactions (Study 1§) Chikungunya-Like Symptom
Chikungunya-Like Symptom (severe)IXCHIQ (N=361)
% (n)Pyrexia (any)
Pyrexia (severe)100 (361)
10.8 (39)Headache (any)
Headache (severe)77.6 (280)
0.3 (1)Fatigue (any)
Fatigue (severe)73.1 (264)
0.6 (2)Myalgia (any)
Myalgia (severe)59.6 (215)
0.8 (3)Arthralgia (any)
Arthralgia (severe)44.0 (159)
1.4 (5)Chills (any)a 8.0 (29) Rash (any)a 6.1 (22) Back pain (any)
Back pain (severe)3.6 (13)
0.3 (1)Lymphadenopathy (any)a 2.5 (9) Dizziness (any)a 1.7 (6) Pain (any)a 1.1 (4) Paresthesia (any)a 0.8 (3) Hyperhidrosis (any)a 0.6 (2) Edema peripheral (any)a 0.6 (2) Asthenia (any)a 0.3 (1) Ataxia (any)a 0.3 (1) Atrial fibrillation (any)
Atrial fibrillation (severe)0.3 (1)
0.3 (1)Feeling abnormal (any)a 0.3 (1) Hypoesthesia (any)a 0.3 (1) Influenza like illness (any)a 0.3 (1) Neuropathy peripheral (any)a 0.3 (1) Rash erythematous (any)a 0.3 (1) Syncope (any)a 0.3 (1) §NCT04546724
N=Number of participants with chikungunya-like adverse reactions; n=number of participants with chikungunya-like symptom.
a No severe chikungunya-like symptoms reported.The median onset of chikungunya-like adverse reactions in IXCHIQ recipients was 4.0 days (range 1 to 11 days) after vaccination. The median duration of chikungunya-like adverse reactions in IXCHIQ recipients was 4.0 days (range 1 day to at least 6 months) after vaccination.
Fourteen IXCHIQ recipients had prolonged (duration at least 30 days) chikungunya-like adverse reactions (median duration 94 days, range 30 days to at least 6 months). Prolonged fatigue, headache and myalgia were each reported by three participants. Prolonged arthralgia was reported by five participants, including a 46-year-old male who reported severe arthralgia and back pain that lasted for at least 51 days after vaccination and a 50-year-old female who reported polyarthralgia and nodular swelling of joints in fingers and foot that lasted for at least 6 months after vaccination.
Two IXCHIQ recipients experienced serious chikungunya-like adverse reactions. A 58-year-old female with a history of fibromyalgia experienced severe myalgia, mild arthralgia, tachycardia and tachypnea, with onset of symptoms 1 to 2 days after vaccination, was hospitalized on Days 4 through 10 post-vaccination, and fully recovered, with myalgia resolving after 30 days. A 66-year-old male experienced severe fever on Days 5 through 11 post-vaccination, was hospitalized on Days 10 through 12 post-vaccination and found to have atrial fibrillation, increased troponin, increased brain natriuretic peptide and hypovolemic hyponatremia, and fully recovered. This study participant was included in a subset of participants assessed for vaccine viremia 7 days after vaccination and was found to be viremic.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to IXCHIQ during pregnancy. Individuals who receive IXCHIQ during pregnancy are encouraged to contact directly, or have their healthcare professional contact, OXON Epidemiology at 1-855-417-6214 to enroll in or obtain information about the registry.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no adequate and well-controlled studies of IXCHIQ in pregnant individuals, and human data available from clinical trials with IXCHIQ are insufficient to establish the presence or absence of vaccine-associated risk during pregnancy.
A developmental study was conducted in female rats. Animals were administered a single human dose of IXCHIQ on 2 occasions, once prior to mating and once during gestation. This study revealed no evidence of harm to the fetus and no adverse effects on post-natal development due to the vaccine [See Animal Data].
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Vertical transmission of wild-type CHIKV to neonates from pregnant individuals with viremia at delivery is common and can cause severe, potentially fatal CHIKV disease in neonates, with neurologic (e.g., encephalopathy, intracranial hemorrhage) and myocardial manifestations. Vertical transmission of wild-type CHIKV and fetal death attributable to CHIKV in the context of antepartum infection has been reported to occur infrequently [See Data].
Fetal/neonatal adverse reactions
Vaccine viremia occurred in the first week following administration of a vaccine containing the same attenuated CHIKV as in IXCHIQ [See Description (11)], with resolution of viremia by 14 days after vaccination [See Clinical Pharmacology (12.2)]. It is not known if the vaccine virus can be transmitted from a pregnant individual to the fetus or neonate and cause fetal or neonatal adverse reactions.
Decisions to administer IXCHIQ during pregnancy should take into consideration the individual’s risk of exposure to wild-type CHIKV, gestational age, and risks to the fetus or neonate from vertical transmission of wild-type CHIKV.
Closely monitor neonates for 7 days after birth for potential disease due to vaccine virus if they are born within 14 days of their mother receiving IXCHIQ.
Data
Human data
In a prospective study conducted during an outbreak of CHIKV, vertical transmission of wild-type CHIKV to neonates from infected pregnant individuals was assessed. Among pregnant individuals infected prepartum (N=22) or intrapartum (N=39) (symptomatic between day -7 and day -3, or day -2 and day 2 around delivery, respectively and concomitant positive serum CHIKV RT-PCR or IgM serology when PCR not available) vertical transmission occurred in 19, all with an intrapartum infection (vertical transmission rate of 48.7% for intrapartum infections). Severe CHIKV disease was reported in 52.6% (10/19) of these infected neonates. Among 678 pregnant individuals infected antepartum (symptomatic between conception and the week preceding labor and positive serum CHIKV RT-PCR or IgM serology) fetal death attributed to CHIKV occurred in three (0.4%). In these three cases, onset of CHIKV symptoms in the pregnant individual ranged from approximately 12 weeks to 15 weeks gestation and the fetal death occurred approximately two weeks later. For these fetal deaths, amniotic fluid before fetal death was CHIKV RT-PCR positive. CHIKV RNA was detected in the placenta and in the fetal brain for two.1
Animal Data
In a pre- and post-natal developmental study with an embryo-fetal development toxicity phase conducted in female rats, a full human dose of IXCHIQ (0.5 mL) was administered by intramuscular injection on 2 occasions to determine the effect on female fertility, reproductive performance, and pre- and post-natal development: 14 days prior to mating, and on gestation day 6. No vaccine related adverse effects on fetal development, reproductive performance, and pre- and post-natal development were reported.
8.2 Lactation
Risk Summary
Human data are not available to assess the impact of IXCHIQ on milk production, its presence in breast milk, or its effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for IXCHIQ and any potential adverse effects on the breastfed child from IXCHIQ or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
Clinical Considerations
Fetal/neonatal adverse reactions
Vaccine viremia occurs after vaccination. In a clinical trial, vaccine virus was not detectable at 14 days after vaccination [See Clinical Pharmacology (12.2)]. The potential for transmission of the vaccine virus from mother to infant through breastmilk is unknown.
Data
Animal Data
One developmental toxicity study was performed in which female rats were administered IXCHIQ at a single dose of up to 300 fold excess relative to the projected human dose (on a mg/kg basis). IXCHIQ-specific antibodies were detected in the milk of vaccinated female rats from day 5 of lactation.
8.4 Pediatric Use
The safety and effectiveness of IXCHIQ in individuals younger than 18 years of age have not been established.
8.5 Geriatric Use
Of the total number of participants in clinical studies of IXCHIQ 9.6% (n=346) were 65 years of age and older, while 1.6% (n=59) were 75 and older. [See Adverse Reactions (6.1) and Clinical Studies (14)]. In Study 1, no overall difference in effectiveness was observed between participants 65 years of age and older and younger participants. Study 1 did not include sufficient numbers of participants 65 years of age and older to determine if there was an overall difference in safety between these participants and younger participants.
-
11 DESCRIPTION
IXCHIQ (Chikungunya Vaccine, Live) is a solution for intramuscular injection. IXCHIQ is supplied as a vial of sterile, Lyophilized Antigen Component, Live, and a syringe of Sterile Water Diluent Component. The Lyophilized Antigen Component, Live, is reconstituted at the time of use with the accompanying Sterile Water Diluent Component to form IXCHIQ. The Lyophilized Antigen Component, Live, is a white to slightly yellowish powder. After reconstitution, IXCHIQ is a clear colorless to slightly yellowish solution.
IXCHIQ contains live, attenuated chikungunya virus (generated by reverse genetics from La Réunion strain LR-CHIKV clone LR2006 OPY1). The attenuated virus has a deletion in non-structural protein 3, which encodes a component of the viral replicase complex, and replicates less efficiently than the wild-type CHIKV. The vaccine virus is propagated in Vero cells (a continuous line of monkey kidney cells) in media containing amino acids, vitamins, minerals and fetal bovine serum. The viral harvests are pooled, clarified and concentrated. The virus is purified by chromatography and ultracentrifugation, mixed with formulation buffer and lyophilized.
After reconstitution, each approximately 0.5-mL dose contains not less than 3.0 log10 TCID50 (Tissue Culture Infectious Dose 50%) of live, attenuated chikungunya virus. Each dose also contains 0.05 mg recombinant human albumin, 25 mg sucrose, 2.5 mg D-sorbitol, 0.75 mg L-methionine, 0.51 mg magnesium chloride hexahydrate, 3.68 mg trisodium citrate di-hydrate, 0.313 mg di-potassium hydrogen phosphate, and 0.098 mg potassium di-hydrogen phosphate. Each dose may contain residual amounts of Vero cell proteins (less than 5 ng/dose), Vero cell DNA (less than 10 pg/dose), bovine serum albumin (less than 500 pg/dose) and protamine sulphate (less than 1 mcg/dose), from the manufacturing process.
IXCHIQ does not contain a preservative.
The stoppers of the syringes containing Sterile Water Diluent Component and the stoppers of the vials containing Lyophilized Antigen Component, Live, are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of protection has not been determined. IXCHIQ elicits CHIKV-specific immune responses.
12.2 Pharmacokinetics
Viremia and urinary shedding
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to assess vaccine viremia in plasma and urinary shedding after vaccination on Days 1 (day of vaccination), 4, 8 and 15 in one Phase 1 study (NCT03382964). Participants were vaccinated with a single dose of one of three dose levels [low (3.5 log10 TCID50/dose), medium (4.5 log10 TCID50/dose), and high (5.5 log10 TCID50/dose)] of a formulation containing the same attenuated CHIKV used in IXCHIQ. The amount of attenuated CHIKV in each medium dose was equivalent to the maximum amount in a dose of IXCHIQ. Viremia was not detected in any participant in any study arm on Day 1. The highest proportion of viremic participants was observed at the Day 4 time point (81%, 90%, and 95% in the low, medium, and high dose groups, respectively, Table 4). Viremia had resolved in all participants by the Day 15 time point, including those who received the high dose formulation. The vaccine virus was detected in the urine of one participant in the low dose group on Day 8.
Table 4. Plasma Viremia on Days 4, 8 and 15 in participants vaccinated with a formulation containing attenuated CHIKV (Study NCT03382964) Visit Attenuated CHIKV Formulation Low1 Dose
(N=31)Medium2 Dose
(N=30)High3 Dose
(N=59)Day 4 Participants with quantifiable viremia, n/N (%) 25 / 31 (81%) 27 / 30 (90%) 56 / 59 (95%) Mean plasma viremia (GCE§/mL) 73,601 89,354 229,224 Day 8 Participants with quantifiable viremia, n/N (%) 6 / 31 (19%) 5 / 30 (17%) 4 / 59 (7%) Mean plasma viremia (GCE§/mL) 8814 15,725 27,028 Day 15 Participants with quantifiable viremia, n/N (%) 0 / 31 (0%) 0 / 30 (0%) 0 / 59 (0%) Mean plasma viremia (GCE§/mL) N/A* N/A* N/A* § GCE = genome copy equivalent; * N/A = not applicable
Dose TCID50: 1low (3.5 log10 TCID50/dose), 2medium (4.5 log10 TCID50/dose), 3high (5.5 log10 TCID50/dose)] -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
IXCHIQ has not been evaluated for carcinogenic or mutagenic potential or impairment of male fertility. In a developmental toxicity study conducted in rats, there were no vaccine-related effects on female fertility [See Use in Specific Populations (8.1)].
13.2 Animal Toxicology and/or Pharmacology
A passive transfer study was performed in non-human primates (NHPs) using human anti-CHIKV immune sera collected from a Phase 1 study (NCT03382964). In the Phase 1 study, participants received a single dose of a vaccine formulation containing the same attenuated CHIKV used in IXCHIQ. Sera obtained between Days 14 and 180 after vaccination were pooled to generate 8 serum pools representing varying anti-CHIKV neutralizing antibody titers. In the passive transfer study, 40 CHIKV-naïve cynomolgus macaques (M. fascicularis) were administered human anti-CHIKV immune sera from the 8 serum pools (n=5 per group) and 6 CHIKV-naïve cynomolgus macaques were administered non-immune control sera by intravenous injection. One day after the transfers, serum samples were obtained from the macaques to determine pre-challenge anti-CHIKV neutralizing antibody titers by μPRNT50 assay. Animals were challenged with 100 times the 50% animal infectious dose of wild-type CHIKV strain La Réunion 2006-OPY1, corresponding to 7,000–10,000 Plaque Forming Units. Animal monitoring included assessment of wild-type CHIKV-induced viremia by RT-qPCR and body temperature through 14 and 28 days after challenge, respectively. None of the animals receiving post-vaccination serum pools developed fever after the challenge. Fever and viremia within 7 days post-challenge were detected in all 6 macaques receiving non-immune human sera. Data from the NHP study were analyzed by logistic regression and a μPRNT50 titer of ≥150 was determined to be reasonably likely to predict clinical benefit in the Phase 3 study.
-
14 CLINICAL STUDIES
IXCHIQ effectiveness against disease caused by CHIKV was based on an evaluation of seroresponse defined as an anti-CHIKV neutralizing antibody level above a threshold (PRNT50 titer 150). This threshold was derived from a non-human primate model [See Nonclinical Toxicology (13.2)].The seroresponse rate 28 days after a single dose of IXCHIQ is presented in Table 5.
Table 5. Seroresponse Rates 28 Days Post-Vaccination as Determined by µPRNT Assay, in Study 1 (PP Population) IXCHIQ Placebo N=266 N=96 % (n) [95%CI] % [95%CI] 98.9 (263) [96.7, 99.8]* 0 [0.0, 3.8] Abbreviations: CI=confidence interval; µPRNT=micro plaque reduction neutralization test; PP=per-protocol (population).
*Success criterion: lower bound of the 95% confidence interval for seroresponse rate >70%.The seroresponse rate 180 days after a single dose of IXCHIQ was 96.3% (95% CI: 93.1, 98.3).
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
IXCHIQ is supplied in a carton (NDC: 42515-003-01) containing:
- one single-dose vial of Lyophilized Antigen Component, Live (NDC: 42515-004-01)
- one prefilled ungraduated syringe of Sterile Water Diluent Component (NDC: 42515-005-01) (packaged without needles).
-
17 PATIENT COUNSELING INFORMATION
Advise the vaccine recipient to read the FDA-approved patient labeling (Patient Information).
Question the vaccine recipient about reactions to previous vaccines.
Inform the vaccine recipient of the benefits and risks of IXCHIQ, including:
- the potential for severe or prolonged chikungunya-like adverse reactions [See Warnings and Precautions (5.2)], and
- the potential for vertical transmission of vaccine virus from pregnant individuals and potential fetal/neonatal adverse reactions [See Warnings and Precautions (5.3)].
Advise the recipient that IXCHIQ may not protect everyone who gets the vaccine and that personal precautions should be taken to reduce exposure to mosquito bites (e.g., adequate clothing, use of repellents, mosquito nets).
There is a pregnancy exposure registry for IXCHIQ. Encourage individuals exposed to IXCHIQ around the time of conception or during pregnancy to register by calling 1-855-417-6214 or by visiting www.valneva-oxon.com/IXCHIQPregnancyRegistry [See Use in Specific Populations (8.1)].
Advise vaccine recipient to report any adverse reactions to their healthcare provider or to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 and www.vaers.hhs.gov.
Manufactured by:
Valneva Scotland Ltd.Oakbank Park Road, EH53 0TG, Livingston, UKT: +44.1506.446.600F: +44.1506.446.601www.valneva.comDistributed by:
Valneva USA Inc.
Bethesda, MD 20814
USA -
Patient Information
IXCHIQ (pronounced “ǐks-chēk”)
Generic name: chikungunya vaccine, live
Read this information about IXCHIQ before you are vaccinated. If you have any questions about IXCHIQ after reading this leaflet, ask your healthcare provider. This leaflet does not take the place of talking with your healthcare professional about IXCHIQ. Only your healthcare provider can decide if IXCHIQ is right for you.
What is IXCHIQ?
IXCHIQ is a vaccine for use in individuals 18 years of age and older to help protect against chikungunya virus disease.
- You should still protect yourself from mosquito bites even if you have received the IXCHIQ vaccine.
- IXCHIQ may not fully protect everyone who gets the vaccine.
- IXCHIQ does not protect against other diseases transmitted by mosquitoes.
Who should not get IXCHIQ?
You should not get IXCHIQ if you:
- have a weakened immune system due to certain conditions or therapy.
- have ever had a severe allergic reaction to any of the ingredients in the vaccine. A list of ingredients can be found at the end of this leaflet.
IXCHIQ is not approved for use in individuals below the age of 18 years.
What should I tell my healthcare professional before I am vaccinated with IXCHIQ?
It is very important to tell your healthcare provider if you:
- have had an allergic reaction to any ingredient of IXCHIQ.
- have a bleeding disorder or are on a blood thinner.
- have a weakened immune system due to a condition or therapy.
- are or may be pregnant, or are breast feeding.
- currently have any illness with a fever of more than 38.0 °C (100.4 °F).
- take any medicines, even those you can buy over the counter.
How is IXCHIQ given?
IXCHIQ is given as an injection into the muscle.
What are the risks of IXCHIQ?
IXCHIQ commonly causes symptoms similar to those experienced by people who have chikungunya disease, e.g., fever, headache, fatigue, muscle pain and joint pain. In some people who receive IXCHIQ, these symptoms may prevent daily activity, require medical intervention including hospitalization, and last for weeks or months.
In some people IXCHIQ causes a decrease in the numbers of white blood cells. This usually occurs within the first week following vaccination. In most people, the numbers of white blood cells return to normal on their own within one month.
The most common side effects of IXCHIQ are headache, fatigue, muscle pain, joint pain, fever, tenderness at the injection site and nausea.
These are not all of the possible side effects of IXCHIQ. You can ask your healthcare provider about other side effects that have been reported.
Contact your healthcare provider right away if you get any symptoms that concern you after receiving IXCHIQ.
Tell your healthcare provider if you have any of the following problems after receiving IXCHIQ because these may be signs of an allergic reaction:
- difficulty breathing
- hoarseness or wheezing
- hives
- dizziness, weakness or fast heart beat
You may report side effects to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or https://vaers.hhs.gov.What are the ingredients of IXCHIQ?
IXCHIQ contains weakened live chikungunya virus (CHIKV), recombinant human albumin, sucrose, D-sorbitol, L-methionine and salts. IXCHIQ may contain trace amounts of bovine serum albumin, Vero cell protein, Vero cell DNA and protamine sulphate.
What else should I know about IXCHIQ?
This leaflet is a summary of information about IXCHIQ. If you would like more information, please talk to your healthcare provider.
11/2023
License Holder:
Valneva Austria GmbH
Campus Vienna Biocenter 31030 Vienna, AustriaManufactured byValneva Scotland Ltd.
Oakbank Park Rd,
Livingston EH53 0TG, United KingdomDistributed by:
Valneva USA Inc.Bethesda, MD 20814USA -
Principal display panel
Text from principal display panel
NDC: 42515-003-01
Rx only
Licence number: 1909
Contains no preservatives
No US standard of potencychikungunya vaccine, live
IXCHIQ
Solution for Intramuscular InjectionFor 18 years of age and older.
Content:- 1 single dose vial of lyophilized vaccine
- 1 single dose prefilled syringe of diluent
-
INGREDIENTS AND APPEARANCE
IXCHIQ
chikungunya vaccine, live-attenuated injection, powder, lyophilized, for solutionProduct Information Product Type VACCINE Item Code (Source) NDC: 42515-003 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHIKUNGUNYA VIRUS LR2006 OPY1 (ATTENUATED STRAIN) ANTIGEN (UNII: 2RM4VT46R8) (CHIKUNGUNYA VIRUS LR2006 OPY1 (ATTENUATED STRAIN) ANTIGEN - UNII:2RM4VT46R8) CHIKUNGUNYA VIRUS LR2006 OPY1 (ATTENUATED STRAIN) ANTIGEN 3 [TCID_50] in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42515-003-01 1 in 1 CARTON 1 NDC: 42515-003-00 0.5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125777 01/15/2024 Labeler - Valneva Scotland Ltd. (737272380) Registrant - Valneva Austria GmbH (300378693) Establishment Name Address ID/FEI Business Operations Valneva Scotland Ltd. 737272380 manufacture, analysis Establishment Name Address ID/FEI Business Operations Valneva Austria GmbH 300378693 analysis
Trademark Results [IXCHIQ]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IXCHIQ 79332212 not registered Live/Pending |
Valneva Austria GmbH 2021-11-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.