ProSys 5000 by Benco Dental ProSys 5000
ProSys 5000 by
Drug Labeling and Warnings
ProSys 5000 by is a Prescription medication manufactured, distributed, or labeled by Benco Dental. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
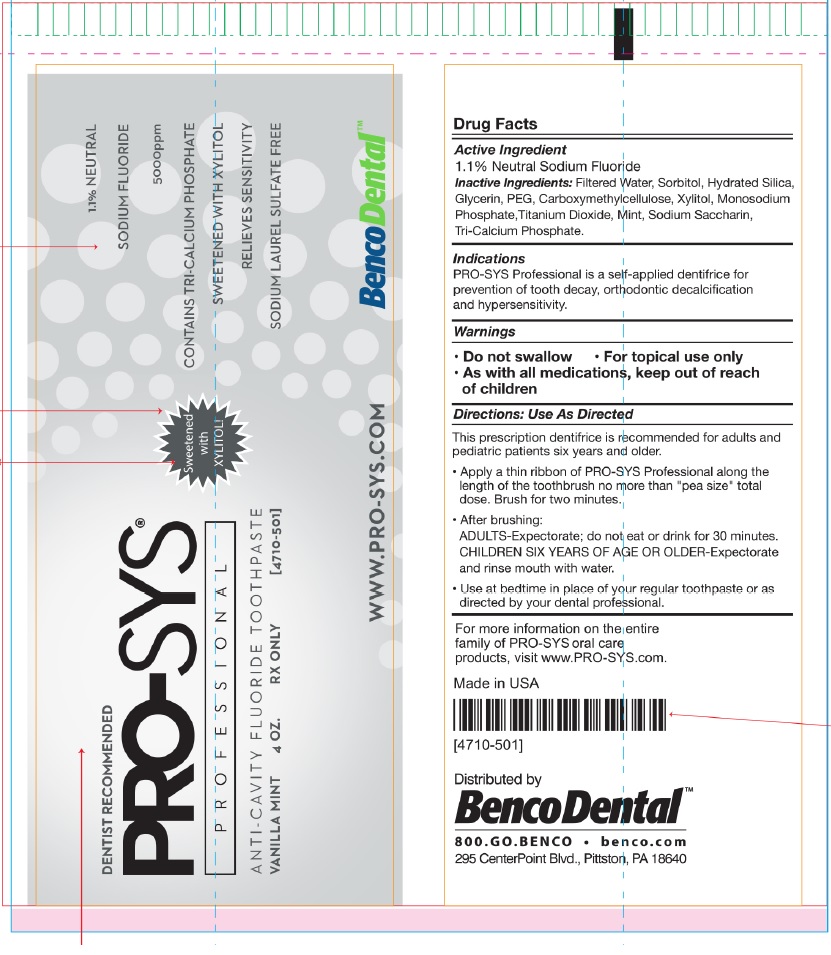
PROSYS 5000- sodium fluoride paste, dentifrice
Benco Dental
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ProSys 5000
Inactive Ingredients:Filtered Water, Sorbitol, Hydrated Silica,
Glycerin, PEG, Carboxymethylcellulose, Xylitol, Monosodium
Phosphate,Titanium Dioxide, Mint, Sodium Saccharin,
Tri-Calcium Phosphate.
Indications
PRO-SYS Professional is a self-applied dentifrice for - prevention of tooth decay, orthodontic decalcification - and hypersensitivity
Directions: Use As Directed
This prescription dentifrice is recommended for adults and
pediatric patients six years and older.
Apply a thin ribbon of PRO-SYS Professional along the
length of the toothbrush no more than "pea size" total
dose. Brush for two minutes.
After brushing:
ADULTS-Expectorate; do not eat or drink for 30 minutes.
CHILDREN SIX YEARS OF AGE OR OLDER-Expectorate
and rinse mouth with water.
Use at bedtime in place of your regular toothpaste or as
directed by your dental professional.
| PROSYS 5000
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Benco Dental (015108087) |
| Registrant - Benco Dental (015108087) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.