These highlights do not include all the information needed to use minocycline hydrochloride extended-release capsules safely and effectively. See full prescribing information for minocycline hydrochloride extended-release capsules.Minocycline hydrochloride extended-release capsules, for oral useInitial U.S. Approval: 1971
Minocycline Hydrochloride by
Drug Labeling and Warnings
Minocycline Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by JG Pharma, Inc., Ohm Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MINOCYCLINE HYDROCHLORIDE- minocycline hydrochloride capsule, extended release
JG Pharma, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use minocycline hydrochloride extended-release capsules safely and effectively. See full prescribing information for minocycline hydrochloride extended-release capsules. Minocycline hydrochloride extended-release capsules, for oral use Initial U.S. Approval: 1971 INDICATIONS AND USAGEMinocycline hydrochloride extended-release capsules is a tetracycline-class drug indicated to treat only inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years of age and older. (1) (1) Limitations of Use (1) Minocycline hydrochloride extended-release capsules did not demonstrate any effect on non-inflammatory acne lesions. Safety of minocycline hydrochloride extended-release capsules has not been established beyond 12 weeks of use. This formulation of minocycline has not been evaluated in the treatment of infections. (14) (1) DOSAGE AND ADMINISTRATIONThe recommended dosage of minocycline hydrochloride extended-release capsules is approximately 1 mg/kg once daily for 12 weeks. (2) (2) DOSAGE FORMS AND STRENGTHSExtended-Release Capsules: 45 mg, 90 mg, and 135 mg (3) (3) CONTRAINDICATIONSMinocycline hydrochloride extended-release capsules is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines. (4) (4) WARNINGS AND PRECAUTIONSThe use of minocycline hydrochloride extended-release capsules during tooth development (last half of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). (5.1) (5) If pseudomembranous colitis occurs, discontinue minocycline hydrochloride extended-release capsules. (5.2) (5) If liver injury is suspected, discontinue minocycline hydrochloride extended-release capsules. (5.3) (5) If renal impairment exists, minocycline hydrochloride extended-release capsules doses may need to be adjusted to avoid excessive systemic accumulations of the drug and possible liver toxicity. (5.4) (5) Minocycline may cause central nervous system side effects including light-headedness, dizziness, or vertigo. Advise patients. (5.5) (5) Minocycline may cause pseudotumor cerebri (benign intracranial hypertension) in adults and adolescents. Discontinue minocycline hydrochloride extended-release capsules if symptoms occur. (5.6) (5) Minocycline has been associated with autoimmune syndromes; discontinue minocycline hydrochloride extended-release capsules immediately if symptoms occur. (5.7) (5) Minocycline has been associated with anaphylaxis, serious skin reactions, erythema multiforme, and DRESS syndrome. Discontinue minocycline hydrochloride extended-release capsules immediately if symptoms occur. (5.9) (5) ADVERSE REACTIONSDRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2023 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Indication
Minocycline hydrochloride extended-release capsules is indicated to treat only inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years of age and older.
To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, minocycline hydrochloride extended-release capsules should be used only as indicated [see Warnings and Precautions (5.11)].
1.2 Limitations of Use
Minocycline hydrochloride extended-release capsules did not demonstrate any effect on non-inflammatory acne lesions. Safety of minocycline hydrochloride extended-release capsules has not been established beyond 12 weeks of use. This formulation of minocycline has not been evaluated in the treatment of infections [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
The recommended dosage of minocycline hydrochloride extended-release capsules is approximately 1 mg/kg once daily for 12 weeks. Higher doses have not shown to be of additional benefit in the treatment of inflammatory lesions of acne, and may be associated with more acute vestibular side effects.
The following table shows capsule strength and body weight to achieve approximately 1 mg/kg.
Table 1: Dosing Table for Minocycline Hydrochloride Extended-Release Capsules
| Patient's Weight (lbs.) | Patient's Weight (kg) | Capsule Strength (mg) | Actual mg/kg Dose |
|---|---|---|---|
|
99 to 131 |
45 to 59 |
45 |
1 to 0.76 |
|
132 to 199 |
60 to 90 |
90 |
1.5 to 1 |
|
200 to 300 |
91 to 136 |
135 |
1.48 to 0.99 |
Minocycline hydrochloride extended-release capsules may be taken with or without food [see Clinical Pharmacology (12.3)]. The capsules should be swallowed whole without chewing, crushing or splitting. Ingestion of food along with minocycline hydrochloride extended-release capsules may help reduce the risk of esophageal irritation and ulceration.
In patients with renal impairment, the total dosage should be decreased by either reducing the recommended individual doses and/or by extending the time intervals between doses [see Warnings and Precautions (5.4)].
3 DOSAGE FORMS AND STRENGTHS
- 45 mg Extended-Release Capsules: Opaque bluish green cap and opaque yellow body hard gelatin capsule with ‘RI18’ imprinted on both cap and body in black ink containing one plain to mottled, yellow to grayish yellow colored film-coated, round tablet plain on both sides.
- 90 mg Extended-Release Capsules: Opaque light blue cap and body hard gelatin capsule with ‘RI19’ imprinted on both cap and body in black ink containing two plain to mottled, yellow to grayish yellow colored film-coated, round tablets plain on both sides.
- 135 mg Extended-Release Capsules: Opaque bluish green cap and opaque light blue body hard gelatin capsule with ‘RI20’ imprinted on both cap and body in black ink containing three plain to mottled, yellow to grayish yellow colored film-coated, round tablets plain on both sides.
4 CONTRAINDICATIONS
Minocycline hydrochloride extended-release capsules is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Teratogenic Effects
A. Minocycline, like other tetracycline-class drugs, can cause fetal harm when administered to a pregnant woman. If any tetracycline is used during pregnancy or if the patient becomes pregnant while taking these drugs, the patient should be apprised of the potential hazard to the fetus.
Minocycline hydrochloride extended-release capsules should not be used during pregnancy or by individuals of either gender who are attempting to conceive a child [see Nonclinical Toxicology (13.1) and Use in Specific Populations (8.1)].
B. The use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown).
Permanent discoloration of the teeth is more common during long-term use of the drug but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. Tetracycline drugs, therefore, should not be used during tooth development.
C. All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every 6 hours. The decrease in fibula growth rate was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can cause retardation of skeletal development on the developing fetus. Evidence of embryotoxicity has been noted in animals treated early in pregnancy [see Use in Specific Populations (8.1)].
5.2 Pseudomembranous Colitis
Clostridium difficile associated diarrhea (CDAD) has been reported with nearly all antibacterial agents, including minocycline, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Hepatotoxicity
Postmarketing cases of serious liver injury, including irreversible drug-induced hepatitis and fulminant hepatic failure (sometimes fatal) have been reported with minocycline use in the treatment of acne.
5.4 Metabolic Effects
The anti-anabolic action of the tetracyclines may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline-class drugs may lead to azotemia, hyperphosphatemia, and acidosis. If renal impairment exists, even usual oral or parenteral doses may lead to excessive systemic accumulations of the drug and possible liver toxicity. Under such conditions, lower than usual total doses are indicated, and if therapy is prolonged, serum level determinations of the drug may be advisable.
5.5 Central Nervous System Effects
Central nervous system side effects including light-headedness, dizziness or vertigo have been reported with minocycline therapy. Patients who experience these symptoms should be cautioned about driving vehicles or using hazardous machinery while on minocycline therapy. These symptoms may disappear during therapy and usually disappear when the drug is discontinued.
5.6 Benign Intracranial Hypertension
Pseudotumor cerebri (benign intracranial hypertension) in adults and adolescents has been associated with the use of tetracyclines. Minocycline has been reported to cause or precipitate pseudotumor cerebri, the hallmark of which is papilledema. Clinical manifestations include headache and blurred vision. Bulging fontanels have been associated with the use of tetracyclines in infants. Although signs and symptoms of pseudotumor cerebri resolve after discontinuation of treatment, the possibility for permanent sequelae such as visual loss that may be severe exists. Patients should be questioned for visual disturbances prior to initiation of treatment with tetracyclines. If visual disturbance occurs during treatment, patients should be checked for papilledema. Concomitant use of isotretinoin and minocycline should be avoided because isotretinoin, a systemic retinoid, is also known to cause pseudotumor cerebri.
5.7 Autoimmune Syndromes
Tetracyclines have been associated with the development of autoimmune syndromes. The long-term use of minocycline in the treatment of acne has been associated with drug-induced lupus-like syndrome, autoimmune hepatitis and vasculitis. Sporadic cases of serum sickness have presented shortly after minocycline use. Symptoms may be manifested by fever, rash, arthralgia, and malaise. In symptomatic patients, liver function tests, ANA, CBC, and other appropriate tests should be performed to evaluate the patients. Use of all tetracycline-class drugs should be discontinued immediately.
5.8 Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines, including minocycline. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using minocycline. If patients need to be outdoors while using minocycline, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician.
5.9 Serious Skin/Hypersensitivity Reaction
Cases of anaphylaxis, serious skin reactions (e.g. Stevens Johnson syndrome), erythema multiforme, and drug rash with eosinophilia and systemic symptoms (DRESS) syndrome have been reported postmarketing with minocycline use in patients with acne. DRESS syndrome consists of cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, and one or more of the following visceral complications such as: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis. Fever and lymphadenopathy may be present. In some cases, death has been reported. If this syndrome is recognized, the drug should be discontinued immediately.
5.10 Tissue Hyperpigmentation
Tetracycline-class antibiotics are known to cause hyperpigmentation. Tetracycline therapy may induce hyperpigmentation in many organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (teeth, mucosa, alveolar bone), sclerae and heart valves. Skin and oral pigmentation has been reported to occur independently of time or amount of drug administration, whereas other tissue pigmentation has been reported to occur upon prolonged administration. Skin pigmentation includes diffuse pigmentation as well as over sites of scars or injury.
5.11 Development of Drug-Resistant Bacteria
Bacterial resistance to the tetracyclines may develop in patients using minocycline hydrochloride extended-release capsules, therefore, the susceptibility of bacteria associated with infection should be considered in selecting antimicrobial therapy. Because of the potential for drug-resistant bacteria to develop during the use of minocycline hydrochloride extended-release capsules, it should be used only as indicated.
5.12 Superinfection
As with other antibiotic preparations, use of minocycline hydrochloride extended-release capsules may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, minocycline hydrochloride extended-release capsules should be discontinued and appropriate therapy instituted.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The following table summarizes selected adverse reactions reported in clinical trials at a rate of ≥ 1% for minocycline hydrochloride.
|
Adverse Reactions |
Minocycline hydrochloride (1 mg/kg) N = 674 (%) |
PLACEBO N = 364 (%) |
|
At least one treatment-emergent event |
379 (56) |
197 (54) |
|
Headache |
152 (23) |
83 (23) |
|
Fatigue |
62 (9) |
24 (7) |
|
Dizziness |
59 (9) |
17 (5) |
|
Pruritus |
31 (5) |
16 (4) |
|
Malaise |
26 (4) |
9 (3) |
|
Mood alteration |
17 (3) |
9 (3) |
|
Somnolence |
13 (2) |
3 (1) |
|
Urticaria |
10 (2) |
1 (0) |
|
Tinnitus |
10 (2) |
5 (1) |
|
Arthralgia |
9 (1) |
2 (0) |
|
Vertigo |
8 (1) |
3 (1) |
|
Dry mouth |
7 (1) |
5 (1) |
|
Myalgia |
7 (1) |
4 (1) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of minocycline hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions that have been reported with minocycline hydrochloride use in a variety of indications include:
Skin and hypersensitivity reactions: fixed drug eruptions, balanitis, erythema multiforme, Stevens-Johnson syndrome, anaphylactoid purpura, photosensitivity, pigmentation of skin and mucous membranes, hypersensitivity reactions, angioneurotic edema, anaphylaxis, DRESS syndrome [see Warnings and Precautions (5.9)].
Autoimmune conditions: polyarthralgia, pericarditis, exacerbation of systemic lupus, pulmonary infiltrates with eosinophilia, transient lupus-like syndrome.
Central nervous system: pseudotumor cerebri, bulging fontanels in infants, decreased hearing.
Endocrine: brown-black microscopic thyroid discoloration, abnormal thyroid function.
Oncology: thyroid cancer.
Oral: glossitis, dysphagia, tooth discoloration.
Gastrointestinal: enterocolitis, pancreatitis, hepatitis, liver failure.
Renal: reversible acute renal failure.
Hematology: hemolytic anemia, thrombocytopenia, eosinophilia.
Preliminary studies suggest that use of minocycline may have deleterious effects on human spermatogenesis [see Nonclinical Toxicology (13.1)].
7 DRUG INTERACTIONS
7.1 Anticoagulants
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
7.2 Penicillin
Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline-class drugs in conjunction with penicillin.
7.3 Methoxyflurane
The concurrent use of tetracycline and methoxyflurane has been reported to result in fatal renal toxicity.
7.4 Antacids and Iron Preparations
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium or magnesium and iron-containing preparations.
7.5 Low Dose Oral Contraceptives
In a multi-center study to evaluate the effect of minocycline hydrochloride (administered as another extended-release formulation which is bioequivalent to minocycline hydrochloride extended-release capsules) on low dose oral contraceptives, hormone levels over one menstrual cycle with and without minocycline hydrochloride 1 mg/kg once-daily were measured. Based on the results of this trial, minocycline-related changes in estradiol, progestinic hormone, FSH and LH plasma levels, of breakthrough bleeding, or of contraceptive failure, cannot be ruled out. To avoid contraceptive failure, female patients are advised to use a second form of contraceptive during treatment with minocycline.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category D [see Warnings and Precautions (5.1)]
Minocycline hydrochloride extended-release capsules should not be used during pregnancy. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus and stop treatment immediately.
There are no adequate and well-controlled studies on the use of minocycline in pregnant women. Minocycline, like other tetracycline-class drugs, crosses the placenta and can cause fetal harm when administered to a pregnant woman.
Spontaneous reports of congenital anomalies including limb reduction have been reported with minocycline use in pregnancy in postmarketing experience. Only limited information is available regarding these reports; therefore, no conclusion on causal association can be established.
Minocycline induced skeletal malformations (bent limb bones) in fetuses when administered to pregnant rats and rabbits in doses of 30 mg/kg/day and 100 mg/kg/day, respectively, (resulting in approximately 3 times and 2 times, respectively, the systemic exposure to minocycline observed in patients as a result of use of minocycline hydrochloride extended-release capsules). Reduced mean fetal body weight was observed in studies in which minocycline was administered to pregnant rats at a dose of 10 mg/kg/day (which resulted in approximately the same level of systemic exposure to minocycline as that observed in patients who use minocycline hydrochloride extended-release capsules).
Minocycline was assessed for effects on peri- and post-natal development of rats in a study that involved oral administration to pregnant rats from day 6 of gestation through the period of lactation (postpartum day 20), at dosages of 5, 10, or 50 mg/kg/day. In this study, body weight gain was significantly reduced in pregnant females that received 50 mg/kg/day (resulting in approximately 2.5 times the systemic exposure to minocycline observed in patients as a result of use of minocycline hydrochloride extended-release capsules). No effects of treatment on the duration of the gestation period or the number of live pups born per litter were observed. Gross external anomalies observed in F1 pups (offspring of animals that received minocycline) included reduced body size, improperly rotated forelimbs, and reduced size of extremities. No effects were observed on the physical development, behavior, learning ability, or reproduction of F1 pups, and there was no effect on gross appearance of F2 pups (offspring of F1 animals).
8.3 Nursing Mothers
Tetracycline-class antibiotics are excreted in human milk. Because of the potential for serious adverse effects on bone and tooth development in nursing infants from the tetracycline-class antibiotics, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother [see Warnings and Precautions (5.1)].
8.4 Pediatric Use
Minocycline hydrochloride extended-release capsules is indicated to treat only inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years and older. Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Use of tetracycline-class antibiotics below the age of 8 is not recommended due to the potential for tooth discoloration [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of minocycline hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
10 OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Minocycline is not removed in significant quantities by hemodialysis or peritoneal dialysis.
11 DESCRIPTION
The active ingredient in minocycline hydrochloride extended-release capsules is minocycline hydrochloride, a semi synthetic derivative of tetracycline. Minocycline hydrochloride extended-release capsules is a tetracycline-class drug. Minocycline hydrochloride extended-release capsules is known chemically as [4 S‑(4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide mono hydrochloride.
The structural formula is represented below:
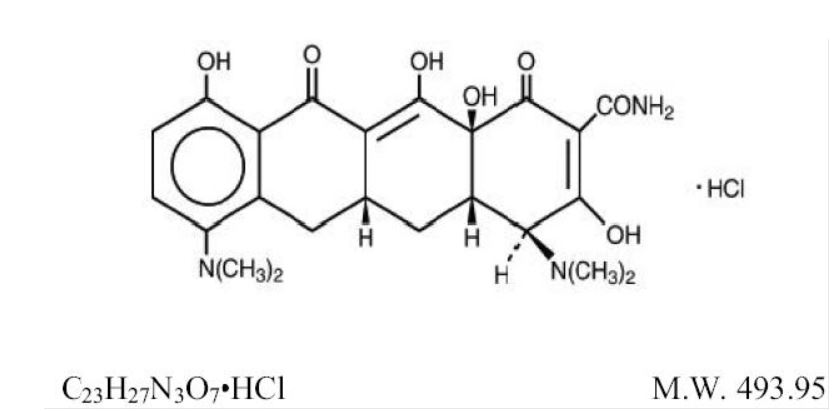
Minocycline hydrochloride, USP is a yellow crystalline powder, sparingly soluble in water, soluble in solutions of alkali hydroxides and carbonates, slightly soluble in alcohol, practically insoluble in chloroform and in ether.
Minocycline hydrochloride extended-release capsules for oral administration contain minocycline hydrochloride, USP equivalent to 45 mg, 90 mg, or 135 mg of minocycline. The extended-release capsules contain the following inactive ingredients: colloidal silicon dioxide, D&C Yellow #10 (in 45 mg strength), FD&C Blue #1, FD&C Yellow #6 (in 45 mg and 135 mg strength), gelatin, hypromellose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, and titanium dioxide.
The 45 mg, 90 mg, and 135 mg capsules also contain Opadry Clear which contains hypromellose, polyethylene glycol 400, polyethylene glycol 6000, and talc.
Minocycline hydrochloride extended-release capsules also contain black ink which contains black iron oxide, potassium hydroxide, propylene glycol, and shellac.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of minocycline hydrochloride extended-release capsules for the treatment of acne is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of minocycline hydrochloride extended-release capsules for the treatment of acne are unknown.
12.3 Pharmacokinetics
Minocycline hydrochloride extended-release capsules is not bioequivalent to immediate release minocycline products.
Following administration of a single dose of minocycline hydrochloride extended-release capsules (135 mg) to 32 healthy male and female adult subjects, the mean (SD) AUC(0-∞) and Cmax were 17.90 (5.56) mcg x hr/mL and 0.96 (0.32) mcg/mL, respectively, under fasting conditions.
In a separate trial, when a single dose of minocycline hydrochloride extended-release capsules (135 mg) was administered with a high fat meal to 30 healthy male and female adult subjects, the mean (SD) AUC(0-∞) and Cmax were 17.16 (3.19) mcg x hr/mL and 0.97 (0.25) mcg/mL, respectively.
Minocycline is lipid soluble and distributes into the skin and sebum.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis-In a carcinogenicity study in which minocycline HCl was orally administered to male and female rats once daily for up to 104 weeks at dosages up to 200 mg/kg/day, minocycline HCl was associated in both genders with follicular cell tumors of the thyroid gland, including increased incidences of adenomas, carcinomas and the combined incidence of adenomas and carcinomas in males, and adenomas and the combined incidence of adenomas and carcinomas in females. In a carcinogenicity study in which minocycline HCl was orally administered to male and female mice once daily for up to 104 weeks at dosages up to 150 mg/kg/day, exposure to minocycline HCl did not result in a significantly increased incidence of neoplasms in either males or females.
Mutagenesis-Minocycline was not mutagenic in vitro in a bacterial reverse mutation assay (Ames test) or CHO/HGPRT mammalian cell assay in the presence or absence of metabolic activation. Minocycline was not clastogenic in vitro using human peripheral blood lymphocytes or in vivo in a mouse micronucleus test.
Impairment of Fertility-Male and female reproductive performance in rats was unaffected by oral doses of minocycline of up to 300 mg/kg/day (which resulted in up to approximately 40 times the level of systemic exposure to minocycline observed in patients as a result of use of minocycline hydrochloride extended-release capsules). However, oral administration of 100 or 300 mg/kg/day of minocycline to male rats (resulting in approximately 15 to 40 times the level of systemic exposure to minocycline observed in patients as a result of use of minocycline hydrochloride extended-release capsules) adversely affected spermatogenesis. Effects observed at 300 mg/kg/day included a reduced number of sperm cells per gram of epididymis, an apparent reduction in the percentage of sperm that were motile, and (at 100 and 300 mg/kg/day) increased numbers of morphologically abnormal sperm cells. Morphological abnormalities observed in sperm samples included absent heads, misshapen heads, and abnormal flagella.
Limited human studies suggest that minocycline may have a deleterious effect on spermatogenesis.
Minocycline hydrochloride extended-release capsules should not be used by individuals of either gender who are attempting to conceive a child.
14 CLINICAL STUDIES
The safety and efficacy of minocycline hydrochloride in the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris was assessed in two 12-week, multi-center, randomized, double-blind, placebo-controlled, trials in subjects ≥ 12 years. The mean age of subjects was 20 years and subjects were from the following racial groups: White (73%), Hispanic (13%), Black (11%), Asian/Pacific Islander (2%), and Other (2%).
In two efficacy and safety trials, a total of 924 subjects with non-nodular moderate to severe acne vulgaris received minocycline hydrochloride or placebo for a total of 12 weeks, according to the following dose assignments.
|
Subject’s Weight (lbs.) |
Subject’s Weight (kg) |
Available Capsule Strength (mg) |
Actual mg/kg Dose |
|
99 to 131 |
45 to 59 |
45 |
1 to 0.76 |
|
132 to 199 |
60 to 90 |
90 |
1.5 to 1 |
|
200 to 300 |
91 to 136 |
135 |
1.48 to 0.99 |
The two primary efficacy endpoints were:
1) Mean percent change in inflammatory lesion counts from Baseline to 12 weeks.
2) Percentage of subjects with an Evaluator’s Global Severity Assessment (EGSA) of clear or almost clear at 12 weeks.
Efficacy results are presented in Table 4.
|
Trial 1 |
Trial 2 |
|||
|
Minocycline hydrochloride (1 mg/kg) N = 300 |
Placebo N = 151 |
Minocycline hydrochloride (1 mg/kg) N = 315 |
Placebo N = 158 |
|
|
Mean Percent Improvement in Inflammatory Lesions |
43.1% |
31.7% |
45.8% |
30.8% |
|
No. (%) of Subjects Clear or Almost Clear on the EGSA* |
52 (17.3%) |
12 (7.9%) |
50 (15.9%) |
15 (9.5%) |
*Evaluator’s Global Severity Assessment
Minocycline hydrochloride did not demonstrate any effect on non-inflammatory lesions (benefit or worsening).
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Minocycline hydrochloride extended-release capsules are hard-gelatin capsules containing minocycline hydrochloride, USP equivalent to 45 mg, 90 mg, or 135 mg minocycline. The extended-release capsules are supplied as follows:
Minocycline hydrochloride extended-release capsules 45 mg: Opaque bluish green cap and opaque yellow body hard gelatin capsule with ‘RI18’ imprinted on both cap and body in black ink containing one plain to mottled, yellow to grayish yellow colored film-coated, round tablet plain on both sides and are supplied as follows:
NDC: 72143-221-30 Bottle of 30
Minocycline hydrochloride extended-release capsules 90 mg: Opaque light blue cap and body hard gelatin capsule with ‘RI19’ imprinted on both cap and body in black ink containing two plain to mottled, yellow to grayish yellow colored film-coated, round tablets plain on both sides and are supplied as follows:
NDC: 72143-222-30 Bottle of 30
Minocycline hydrochloride extended-release capsules 135 mg: Opaque bluish green cap and opaque light blue body hard gelatin capsule with ‘RI20’ imprinted on both cap and body in black ink containing three plain to mottled, yellow to grayish yellow colored film-coated, round tablets plain on both sides and are supplied as follows:
NDC: 72143-223-30 Bottle of 30
17 PATIENT COUNSELING INFORMATION
“See FDA-Approved Patient Labeling (Patient Information)”
Patients taking minocycline hydrochloride extended-release capsules should receive the following information and instructions:
- Minocycline hydrochloride extended-release capsules should not be used by pregnant women or women attempting to conceive a child [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)].
- It is recommended that minocycline hydrochloride extended-release capsules not be used by men who are attempting to father a child [see Nonclinical Toxicology (13.1)].
- Patients should be advised that pseudomembranous colitis can occur with minocycline therapy. If patients develop watery or bloody stools, they should seek medical attention.
- Patients should be counseled about the possibility of hepatotoxicity. Patients should seek medical advice if they experience symptoms which can include loss of appetite, tiredness, diarrhea, skin turning yellow, bleeding easily, confusion, and sleepiness.
- Patients who experience central nervous system symptoms [see Warnings and Precautions (5.5)] should be cautioned about driving vehicles or using hazardous machinery while on minocycline therapy. Patients should seek medical help for persistent headaches or blurred vision.
- Concurrent use of tetracycline may render oral contraceptives less effective. To avoid contraceptive failure, female patients on low dose oral contraceptives should be advised to use a second form of contraception during treatment with minocycline [see Drug Interactions (7.5)].
- Autoimmune syndromes, including drug-induced lupus-like syndrome, autoimmune hepatitis, vasculitis and serum sickness have been observed with tetracycline-class drugs, including minocycline. Symptoms may be manifested by arthralgia, fever, rash and malaise. Patients who experience such symptoms should be cautioned to stop the drug immediately and seek medical help.
- Patients should be counseled about discoloration of skin, scars, teeth or gums that can arise from minocycline therapy.
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines, including minocycline. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using minocycline. If patients need to be outdoors while using minocycline, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Treatment should be discontinued at the first evidence of skin erythema.
- Minocycline hydrochloride extended-release capsules should be taken exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the current treatment course and increase the likelihood that bacteria will develop resistance and will not be treatable by other antibacterial drugs in the future.
- Patients should be advised to swallow minocycline hydrochloride extended-release capsules whole and not to chew, crush, or split the capsules.
Manufactured by:
Ohm Laboratories Inc.
New Brunswick, NJ 08901
Distributed by:
JG Pharma
Scottsdale, AZ 85258
Issued July 2020 FDA-12
FDA-APPROVED PATIENT LABELING
PATIENT INFORMATION
Minocycline Hydrochloride
(min-oh-SY-kleen hy-droh-KLOR-ide)
Extended-Release Capsules
Read this Patient Information leaflet that comes with minocycline hydrochloride extended-release capsules before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or treatment.
What is minocycline hydrochloride extended-release capsules?
Minocycline hydrochloride extended-release capsules is a tetracycline-class drug. Minocycline hydrochloride extended-release capsules is prescription medicine used to treat pimples and red bumps (non-nodular inflammatory lesions) that happen with moderate to severe acne vulgaris in people 12 years and older. Minocycline hydrochloride extended-release capsules is not effective for acne that is not red-looking (this means acne that is not inflammatory).
It is not known if minocycline hydrochloride extended-release capsules is:
- safe for use longer than 12 weeks.
- safe and effective for the treatment of infections.
- safe and effective in children under the age of 12 years.
Who should not take minocycline hydrochloride extended-release capsules?
Do not take minocycline hydrochloride extended-release capsules if you are allergic to tetracycline class medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
What should I tell my doctor before taking minocycline hydrochloride extended-release capsules?
Before you take minocycline hydrochloride extended-release capsules, tell your doctor if you:
- have kidney problems. Your doctor may prescribe a lower dose of medicine for you
- have liver problems
- have diarrhea or watery stools
- have vision problems
- plan to have surgery with general anesthesia
- have any other medical conditions
- are a male, and you and your female partner are trying to conceive a baby. You should not take minocycline hydrochloride extended-release capsules.
- are pregnant or plan to become pregnant. Minocycline hydrochloride extended-release capsules may harm your unborn baby. Taking minocycline hydrochloride extended-release capsules while you are pregnant may cause serious side effects on the growth of bone and teeth of your baby. Talk to your doctor before taking minocycline hydrochloride extended-release capsules if you plan to become pregnant, or if you are already taking minocycline hydrochloride extended-release capsules and plan to become pregnant. Stop taking minocycline hydrochloride extended-release capsules and call your doctor right away if you become pregnant while taking minocycline hydrochloride extended-release capsules.
- are breastfeeding or plan to breastfeed. minocycline hydrochloride extended-release capsules passes into your milk and may harm your baby. You and your doctor should decide if you will take minocycline hydrochloride extended-release capsules or breastfeed. You should not do both.
Tell your doctor about all the other medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Minocycline hydrochloride extended-release capsules may affect the way other medicines work, and other medicines may affect how minocycline hydrochloride extended-release capsules works.
Especially tell your doctor if you take:
- birth control pills. Minocycline hydrochloride extended-release capsules may make your birth control pills less effective. You could become pregnant. You should use a second form of birth control while taking minocycline hydrochloride extended-release capsules.
- a blood thinner medicine.
- a penicillin antibiotic medicine. Minocycline hydrochloride extended-release capsules and penicillins should not be used together.
- antacids that contain aluminum, calcium, or magnesium or iron-containing products.
- an acne medication that contains isotretinoin. Minocycline hydrochloride extended-release capsules and isotretinoin should not be used together.
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take minocycline hydrochloride extended-release capsules?
- Take minocycline hydrochloride extended-release capsules exactly as your doctor tells you.
- Skipping doses or not taking all doses of minocycline hydrochloride extended-release capsules may:
make the treatment not work as well.
increase the chance that the bacteria will become resistant to minocycline hydrochloride extended-release capsules.
- Minocycline hydrochloride extended-release capsules can be taken with or without food. Taking minocycline hydrochloride extended-release capsules with food may lower your chances of getting irritation or ulcers in your esophagus. Your esophagus is the tube that connects your mouth to your stomach.
- Swallow minocycline hydrochloride extended-release capsules whole. Do not chew, crush, or split the capsules.
If you take too much minocycline hydrochloride extended-release capsules, call your doctor or poison control center right away. Your doctor may do blood tests to check you for side effects during treatment with minocycline hydrochloride extended-release capsules.
What should I avoid while taking minocycline hydrochloride extended-release capsules?
- Avoid sunlight, sunlamps, and tanning beds. Minocycline hydrochloride extended-release capsules can make your skin sensitive to the sun and the light from sunlamps and tanning beds. You could get severe sunburn.
- Wear loose-fitting clothes that protect your skin from sun exposure. Talk to your doctor about other ways to protect your skin while out in sunlight.
- You should not drive or operate dangerous machinery until you know how minocycline hydrochloride extended-release capsules affects you. Minocycline hydrochloride extended-release capsules may cause you to feel dizzy or lightheaded, or have a spinning feeling (vertigo).
What are possible side effects of minocycline hydrochloride extended-release capsules?
Minocycline hydrochloride extended-release capsules may cause serious side effects, including:
Harm to an unborn baby. See “What should I tell my doctor before taking minocycline hydrochloride extended-release capsules?”
- Permanent teeth discoloration. Minocycline hydrochloride extended-release capsules may permanently turn a baby or child's teeth yellow-grey-brown during tooth development. Minocycline hydrochloride extended-release capsules should not be used during tooth development. Tooth development happens in the last half of pregnancy, and from birth to 8 years of age. See “What should I tell my doctor before taking minocycline hydrochloride extended-release capsules?”
- Intestine infection (pseudomembranous colitis). Pseudomembranous colitis can happen with most antibiotics, including minocycline hydrochloride extended-release capsules. Call your doctor right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools. You may have stomach cramps and a fever. Pseudomembranous colitis can happen 2 or more months after you have finished your medication.
- Serious liver problems. Stop taking minocycline hydrochloride extended-release capsules and call your doctor right away if you get any of the following symptoms of liver problems:
loss of appetite
tiredness
diarrhea
yellowing of your skin or the whites of your eyes
unexplained bleeding
confusion
sleepiness
- Central nervous system effects. See “What should I avoid while taking minocycline hydrochloride extended-release capsules?” Central nervous system effects such as light headedness, dizziness, and a spinning feeling (vertigo) may go away during your treatment with minocycline hydrochloride extended-release capsules or if treatment is stopped. Call your doctor if you get headaches that do not go away or blurred vision.
- Benign intracranial hypertension, also called pseudotumor cerebri. This is a condition where there is high pressure in the fluid around the brain. This swelling may lead to vision changes and permanent vision loss. Stop taking minocycline hydrochloride extended-release capsules and tell your doctor right away if you have blurred vision, vision loss, or unusual headaches.
- Immune system reactions including a lupus-like syndrome, hepatitis, and inflammation of blood or lymph vessels (vasculitis). Using minocycline hydrochloride extended-release capsules for a long time to treat acne may cause immune system reactions. Tell your doctor right away if you get a fever, rash, joint pain, or body weakness. Your doctor may do tests to check your blood for immune system reactions.
- Serious rash and allergic reactions. Minocycline hydrochloride extended-release capsules may cause a serious rash and allergic reactions that may affect parts of your body such as your liver, lungs, kidneys and heart. Sometimes these can lead to death.
- Stop taking minocycline hydrochloride extended-release capsules and get medical help right away if you have any of these symptoms:
skin rash, hives, sores in your mouth, or your skin blisters and peels
swelling of your face, eyes, lips, tongue, or throat
trouble swallowing or breathing
blood in your urine
fever, yellowing of the skin or the whites of your eyes, dark colored urine
pain on the right side of the stomach area (abdominal pain)
chest pain or abnormal heartbeats
swelling in your legs, ankles, and feet
darkening of your nails, skin, eyes, scars, teeth, and gums
The most common side effects of minocycline hydrochloride extended-release capsules include:
- headache
- tiredness
- dizziness or spinning feeling
- itching
Call your doctor if you have a side effect that bothers you or that does not go away. Your doctor may do tests to check you for side effects during treatment with minocycline hydrochloride extended-release capsules.
These are not all the side effects with minocycline hydrochloride extended-release capsules. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store minocycline hydrochloride extended-release capsules?
- Store minocycline hydrochloride extended-release capsules at room temperature between 68º F to 77º F (20º C to 25º C).
- Keep minocycline hydrochloride extended-release capsules in the container that it comes in and keep the container tightly closed.
- Keep minocycline hydrochloride extended-release capsules dry.
Keep minocycline hydrochloride extended-release capsules and all medicines out of the reach of children.
General information about minocycline hydrochloride extended-release capsules
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use minocycline hydrochloride extended-release capsules for a condition for which it was not prescribed. Do not give minocycline hydrochloride extended-release capsules to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about minocycline hydrochloride extended-release capsules. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about minocycline hydrochloride extended-release capsules that is written for health professionals.
What are the ingredients in minocycline hydrochloride extended-release capsules?
Active Ingredient: minocycline HCl, USP.
Inactive Ingredients: colloidal silicon dioxide, D&C Yellow #10 (in 45 mg strength), FD&C Blue #1, FD&C Yellow #6 (in 45 mg and 135 mg strength), gelatin, hypromellose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, and titanium dioxide.
The 45 mg, 90 mg, and 135 mg capsules also contain Opadry Clear which contains hypromellose, polyethylene glycol 400, polyethylene glycol 6000, and talc.
Minocycline hydrochloride extended-release capsules also contains black ink which contains black iron oxide, potassium hydroxide, propylene glycol, and shellac.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Ohm Laboratories Inc.
New Brunswick, NJ 08901
Distributed by:
JG Pharma
Scottsdale, AZ 85258
Issued July 2020 FDA-12
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - bottle label (45 mg - 30s)
NDC 72143-221-30
Minocycline
Hydrochloride
Extended-Release
Capsules
45 mg*
Rx only 30 Capsules

Package/Label Display Panel - bottle label (90 mg - 30s)
NDC: 72143-222-30
Minocycline
Hydrochloride
Extended-Release
Capsules
90 mg*
Rx only 30 Capsules

Package/Label Display Panel- bottle label (135 mg - 30s)
NDC: 72143-223-30
Minocycline
Hydrochloride
Extended-Release
Capsules
135 mg*
Rx only 30 Capsules

| MINOCYCLINE HYDROCHLORIDE
minocycline hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| MINOCYCLINE HYDROCHLORIDE
minocycline hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| MINOCYCLINE HYDROCHLORIDE
minocycline hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - JG Pharma, Inc. (081048334) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ohm Laboratories Inc. | 184769029 | manufacture(72143-221, 72143-222, 72143-223) , pack(72143-221, 72143-222, 72143-223) , analysis(72143-221, 72143-222, 72143-223) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.