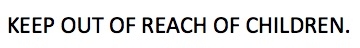GINGI-AID SOLUTION- aluminum chloride 25% solution
Gingi-Aid Solution by
Drug Labeling and Warnings
Gingi-Aid Solution by is a Otc medication manufactured, distributed, or labeled by Gingi-Pak a Division of the Belport. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Warnings
- Precautions
- Purpose
- Storage
- Uses
- Description
- Contraindications
- Keep out of reach of children
- Direction
- Inactive ingredients
- Principal display
-
INGREDIENTS AND APPEARANCE
GINGI-AID SOLUTION
aluminum chloride 25% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10129-017 Route of Administration DENTAL, ORAL, PERIODONTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLORIDE (UNII: 3CYT62D3GA) (ALUMINUM CATION - UNII:3XHB1D032B) ALUMINUM CHLORIDE 3.75 g in 15 mL Inactive Ingredients Ingredient Name Strength CHLOROBUTANOL (UNII: HM4YQM8WRC) 3 mg in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10129-017-02 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/04/1990 2 NDC: 10129-017-03 40 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/04/1990 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 04/04/1990 Labeler - Gingi-Pak a Division of the Belport (008480121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.