ALCOHOL DISINFECTING WIPE- alcohol swab
Alcohol Disinfecting Wipe by
Drug Labeling and Warnings
Alcohol Disinfecting Wipe by is a Otc medication manufactured, distributed, or labeled by McKesson Medical-Surgical, Jiangsu Province Tech (Shanghai). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
-
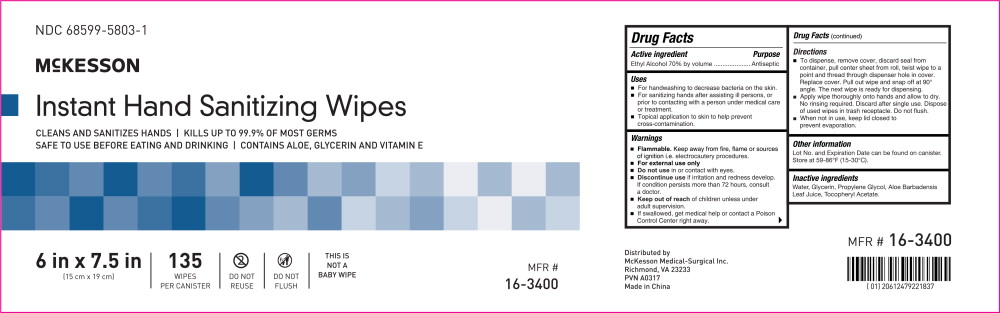
Principal Display Panel - Canister Label
NDC: 68599-5803-1
McKESSON
Instant Hand Sanitizing Wipes
CLEANS AND SANITIZES HANDS | KILLS UP TO 99.9% OF MOST GERMS
SAFE TO USE BEFORE EATING AND DRINKING | CONTAINS ALOE, GLYCERIN AND VITAMIN E
6 in x 7.5 in
(15 cm x 19 cm)
135
WIPES
PER CANISTERDO NOT
REUSEDO NOT
FLUSHTHIS IS
NOT A
BABY WIPEMFR # 16-3400
-
Principal Display Panel - Canister Case Label
NDC: 68599-5803-2
16-3400
McKESSON
Alcohol Disinfecting
Hand WipesCLEANS AND SANITIZES HANDS | KILLS UP TO 99.9% OF MOST GERMS
SAFE TO USE BEFORE EATING AND DRINKING | CONTAINS ALOE, GLYERIN AND VITAMIN E
6 in x 7.5 in
(15 cm x 19 cm)
135
WIPES
PER CANISTER12
CANISTERS
PER CASEDO NOT REUSE
DO NOT FLUSH
THIS IS NOT A BABY WIPE
Store at 59-86°F (15-30°).
Distributed by
McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0317
Made in China
MFR # 16-3400
-
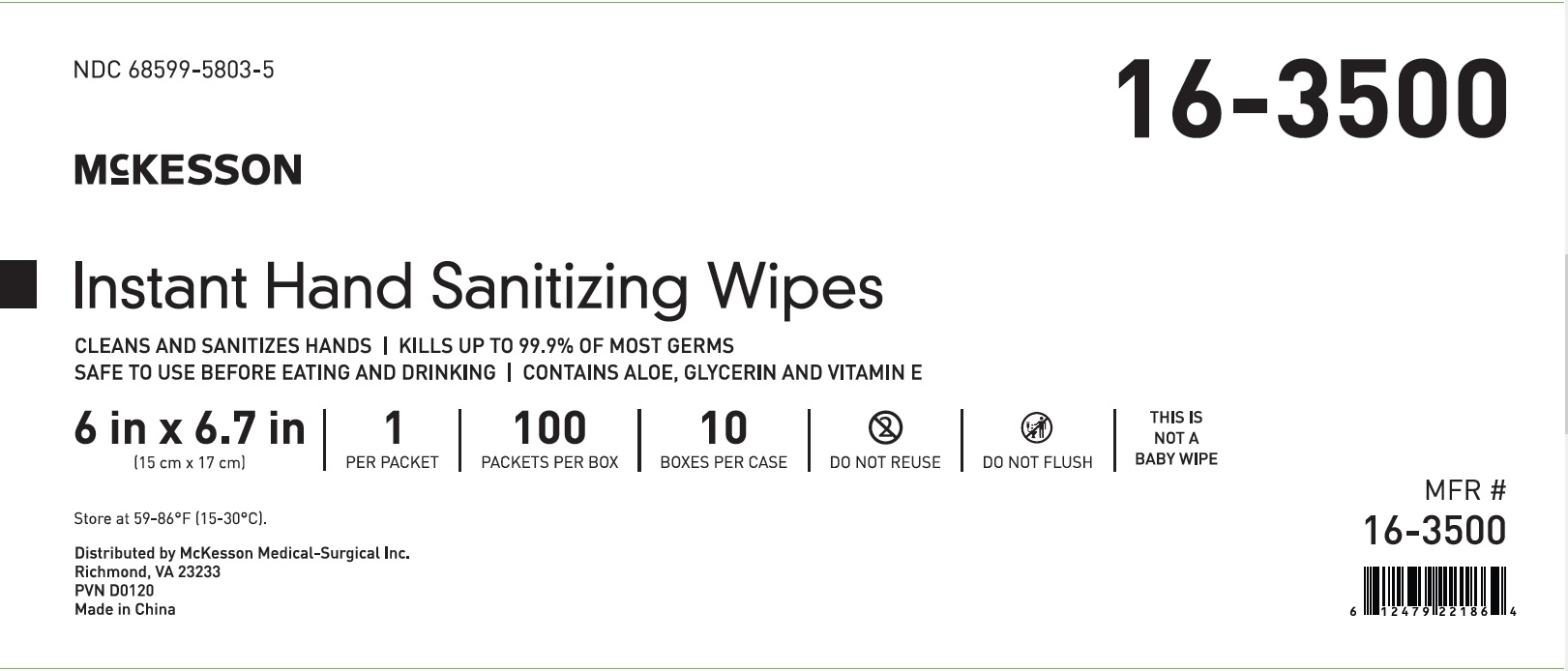
Principal Display Panel - Packet Carton Label
NDC: 68599-5803-3
McKESSON
Instant Hand
Sanitizing WipesCLEANS AND SANITIZES HANDS
KILLS UP TO 99.9% OF MOST GERMS
SAFE TO USE BEFORE EATING AND DRINKING
CONTAINS ALOE, GLYCERIN AND VITAMIN E
6 in x 6.7 in
(15 cm x 17 cm)
1
PER
PACKET100
PACKETS
PER BOXDO NOT
REUSEDO NOT
FLUSHTHIS IS
NOT A
BABY WIPEMFR #
16-3500 -
Principal Display Panel - Packet Case Label
NDC: 68599-5803-4
McKESSON
Instant Hand Sanitizing Wipes
CLEANS AND SANITIZES HANDS | KILLS UP TO 99.9% OF MOST GERMS
SAFE TO USE BEFORE EATING AND DRINKING | CONTAINS ALOE, GLYCERIN AND VITAMIN E
6 in x 6.7 in
(15 cm x 17 cm)
1 PER PACKET
100 PACKETS PER BOX
10 BOXES PER CASE
DO NOT REUSE
DO NOT FLUSH
THIS IS NOT A BABY WIPE
Store at 59-86°F (15-30°C).
Distributed by McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0317
Made in China
MFR # 16-3500


- Principal Display Panel - Packet Label
-
INGREDIENTS AND APPEARANCE
ALCOHOL DISINFECTING WIPE
alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68599-5803 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE (UNII: V5VD430YW9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68599-5803-2 12 in 1 CASE 07/01/2017 1 NDC: 68599-5803-1 135 in 1 CANISTER; Type 0: Not a Combination Product 2 NDC: 68599-5803-4 10 in 1 CASE 07/01/2017 2 NDC: 68599-5803-3 100 in 1 BOX 2 NDC: 68599-5803-5 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/01/2017 Labeler - McKesson Medical-Surgical (023904428) Establishment Name Address ID/FEI Business Operations Jiangsu Province Tech (Shanghai) 530968767 manufacture(68599-5803)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.