CYCLOBENZAPRINE HYDROCHLORIDE tablet, film coated
Cyclobenzaprine Hydrochloride by
Drug Labeling and Warnings
Cyclobenzaprine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by AvPAK. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
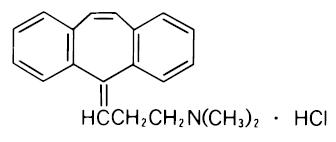
Cyclobenzaprine hydrochloride, USP is a white, crystalline tricyclic amine salt with the empirical formula C 20H 21NHCl and a molecular weight of 311.9. It has a melting point of 217°C, and a pK a of 8.47 at 25°C. It is freely soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base separates. Cyclobenzaprine hydrochloride, USP is designated chemically as 3-( 5H –dibenzo[ a,d]cyclohepten-5-ylidene)- N, N-dimethyl-1-propanamine hydrochloride, and has the following structural formula:
Cyclobenzaprine HCl USP, 5 mg is supplied as a 5 mg tablet for oral administration.
Cyclobenzaprine HCl USP, 10 mg is supplied as a 10 mg tablet for oral administration.
Cyclobenzaprine HCl tablets USP, 5 mg contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, pregelatinized starch, colloidal silicon dioxide, magnesium stearate and opadry beige (hypromellose 6cP, titanium dioxide, PEG 400, iron oxide yellow and iron oxide red).
Cyclobenzaprine HCl tablets USP, 10 mg contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, pregelatinized starch, colloidal silicon dioxide, magnesium stearate and opadry yellow (hypromellose 3cp, hypromellose 6cp, titanium dioxide, PEG 400, iron oxide yellow and polysorbate 80).
-
CLINICAL PHARMACOLOGY
Cyclobenzaprine HCl relieves skeletal muscle spasm of local origin without interfering with muscle function. It is ineffective in muscle spasm due to central nervous system disease.
Cyclobenzaprine reduced or abolished skeletal muscle hyperactivity in several animal models. Animal studies indicate that cyclobenzaprine does not act at the neuromuscular junction or directly on skeletal muscle. Such studies show that cyclobenzaprine acts primarily within the central nervous system at brain stem as opposed to spinal cord levels, although its action on the latter may contribute to its overall skeletal muscle relaxant activity. Evidence suggests that the net effect of cyclobenzaprine is a reduction of tonic somatic motor activity, influencing both gamma (γ) and alpha (α) motor systems.
Pharmacological studies in animals showed a similarity between the effects of cyclobenzaprine and the structurally related tricyclic antidepressants, including reserpine antagonism, norepinephrine potentiation, potent peripheral and central anticholinergic effects, and sedation. Cyclobenzaprine caused slight to moderate increase in heart rate in animals.
Pharmacokinetics
Estimates of mean oral bioavailability of cyclobenzaprine range from 33% to 55%. Cyclobenzaprine exhibits linear pharmacokinetics over the dose range 2.5 mg to 10 mg, and is subject to enterohepatic circulation. It is highly bound to plasma proteins. Drug accumulates when dosed three times a day, reaching steady-state within 3-4 days at plasma concentrations about four-fold higher than after a single dose. At steady state in healthy subjects receiving 10 mg t.i.d. (n=18), peak plasma concentration was 25.9 ng/mL (range, 12.8-46.1 ng/mL), and area under the concentration-time (AUC) curve over an 8-hour dosing interval was 177 ng.hr/mL (range, 80-319 ng.hr/mL).
Cyclobenzaprine is extensively metabolized, and is excreted primarily as glucuronides via the kidney. Cytochromes P-450 3A4, 1A2, and, to a lesser extent, 2D6, mediate N-demethylation, one of the oxidative pathways for cyclobenzaprine. Cyclobenzaprine is eliminated quite slowly, with an effective half-life of 18 hours (range 8-37 hours; n=18); plasma clearance is 0.7 L/min.
The plasma concentration of cyclobenzaprine is generally higher in the elderly and in patients with hepatic impairment. (See PRECAUTIONS, Use in the Elderly and PRECAUTIONS, Impaired Hepatic Function.)
Elderly
In a pharmacokinetic study in elderly individuals (≥65yrs old), mean (n=10) steady-state cyclobenzaprine AUC values were approximately 1.7 fold (171.0 ng.hr/mL, range 96.1-255.3) higher than those seen in a group of eighteen younger adults (101.4 ng.hr/mL, range 36.1-182.9) from another study. Elderly male subjects had the highest observed mean increase, approximately 2.4 fold (198.3 ng.hr/mL, range 155.6-255.3 versus 83.2 ng.hr/mL, range 41.1-142.5 for younger males) while levels in elderly females were increased to a much lesser extent, approximately 1.2 fold (143.8 ng.hr/mL, range 96.1-196.3 versus 115.9 ng.hr/mL, range 36.1-182.9 for younger females).
In light of these findings, therapy with cyclobenzaprine HCl in the elderly should be initiated with a 5 mg dose and titrated slowly upward.
Hepatic Impairment
In a pharmacokinetic study of sixteen subjects with hepatic impairment (15 mild, 1 moderate per Child-Pugh score), both AUC and C max were approximately double the values seen in the healthy control group. Based on the findings, cyclobenzaprine HCl should be used with caution in subjects with mild hepatic impairment starting with the 5 mg dose and titrating slowly upward. Due to the lack of data in subjects with more severe hepatic insufficiency, the use of cyclobenzaprine HCl in subjects with moderate to severe impairment is not recommended.
No significant effect on plasma levels or bioavailability of cyclobenzaprine HCl or aspirin was noted when single or multiple doses of the two drugs were administered concomitantly. Concomitant administration of cyclobenzaprine HCl and naproxen or diflunisal was well tolerated with no reported unexpected adverse effects. However combination therapy of cyclobenzaprine HCl with naproxen was associated with more side effects than therapy with naproxen alone, primarily in the form of drowsiness. No well-controlled studies have been performed to indicate that cyclobenzaprine HCl enhances the clinical effect of aspirin or other analgesics, or whether analgesics enhance the clinical effect of cyclobenzaprine HCI in acute musculoskeletal conditions.
Clinical Studies
Eight double-blind controlled clinical studies were performed in 642 patients comparing cyclobenzaprine HCl 10 mg, diazepam**, and placebo. Muscle spasm, local pain and tenderness, limitation of motion, and restriction in activities of daily living were evaluated. In three of these studies there was a significantly greater improvement with cyclobenzaprine HCl than with diazepam, while in the other studies the improvement following both treatments was comparable.
Although the frequency and severity of adverse reactions observed in patients treated with cyclobenzaprine HCl were comparable to those observed in patients treated with diazepam, dry mouth was observed more frequently in patients treated with cyclobenzaprine HCl and dizziness more frequently in those treated with diazepam. The incidence of drowsiness, the most frequent adverse reaction, was similar with both drugs.
The efficacy of cyclobenzaprine HCl tablets, 5 mg was demonstrated in two seven-day, double-blind, controlled clinical trials enrolling 1405 patients. One study compared cyclobenzaprine HCl tablets, 5 mg and 10 mg t.i.d. to placebo; and a second study compared cyclobenzaprine HCl tablets, 5 mg and 2.5 mg t.i.d. to placebo. Primary endpoints for both trials were determined by patient-generated data and included global impression of change, medication helpfulness, and relief from starting backache. Each endpoint consisted of a score on a 5-point rating scale (from 0 or worst outcome to 4 or best outcome). Secondary endpoints included a physician's evaluation of the presence and extent of palpable muscle spasm.
Comparisons of cyclobenzaprine HCl tablets, 5 mg and placebo groups in both trials established the statistically significant superiority of the 5 mg dose for all three primary endpoints at day 8 and, in the study comparing 5 and 10 mg, at day 3 or 4 as well. A similar effect was observed with cyclobenzaprine HCl tablets, 10 mg (all endpoints). Physician-assessed secondary endpoints also showed that cyclobenzaprine HCl tablets, 5 mg was associated with a greater reduction in palpable muscle spasm than placebo.
Analysis of the data from controlled studies shows that cyclobenzaprine HCl produces clinical improvement whether or not sedation occurs.
** VALIUM® (diazepam, Roche)
Surveillance Program
A post-marketing surveillance program was carried out in 7607 patients with acute musculoskeletal disorders, and included 297 patients treated with cyclobenzaprine HCl tablets, 10 mg for 30 days or longer. The overall effectiveness of cyclobenzaprine HCl was similar to that observed in the double-blind controlled studies; the overall incidence of adverse effects was less (see ADVERSE REACTIONS).
-
INDICATIONS AND USAGE
Cyclobenzaprine HCl tablets USP are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions.
Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, limitation of motion, and restriction in activities of daily living.
Cyclobenzaprine HCl should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
cyclobenzaprine HCl has not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease, or in children with cerebral palsy.
-
CONTRAINDICATIONS
Hypersensitivity to any component of this product.
Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures, and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
Acute recovery phase of myocardial infarction, and patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
Hyperthyroidism.
-
WARNINGS
Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with Cyclobenzaprine HCI when used in combination with other drugs, such as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. The concomitant use of cyclobenzaprine HCI with MAO inhibitors is contraindicated (see CONTRAINDICATIONS). Serotonin syndrome symptoms may include mental status changes (e.g., confusion, agitation, hallucinations), autonomic instability (e.g., diaphoresis, tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., tremor, ataxia, hyperreflexia, clonus, muscle rigidity), and /or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Treatment with Cyclobenzaprine HCI and any concomitant serotonergic agents should be discontinued immediately if the above reactions occur and supportive symptomatic treatment should be initiated. If concomitant treatment with Cyclobenzaprine HCI and other serotonergic drugs is clinically warranted, careful observation is advised, particularly during treatment initiation or dose increases(see PRECAUTIONS, Drug Interactions).
Cyclobenzaprine is closely related to the tricyclic antidepressants, e.g., amitriptyline and imipramine. In short term studies for indications other than muscle spasm associated with acute musculoskeletal conditions, and usually at doses somewhat greater than those recommended for skeletal muscle spasm, some of the more serious central nervous system reactions noted with the tricyclic antidepressants have occurred (see WARNINGS, below, and ADVERSE REACTIONS).Tricyclic antidepressants have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke.
Cyclobenzaprine HCl may enhance the effects of alcohol, barbiturates, and other CNS depressants.
-
PRECAUTIONS
General
Because of its atropine-like action, cyclobenzaprine HCl should be used with caution in patients with a history of urinary retention, angle-closure glaucoma, increased intraocular pressure, and in patients taking anticholinergic medication.
Impaired Hepatic Function
The plasma concentration of cyclobenzaprine is increased in patients with hepatic impairment (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Hepatic Impairment). These patients are generally more susceptible to drugs with potentially sedating effects, including cyclobenzaprine. Cyclobenzaprine HCl tablets should be used with caution in subjects with mild hepatic impairment starting with a 5 mg dose and titrating slowly upward. Due to the lack of data in subjects with more severe hepatic insufficiency, the use of cyclobenzaprine HCl in subjects with moderate to severe impairment is not recommended.
Information for Patients
Cyclobenzaprine HCl, especially when used with alcohol or other CNS depressants, may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. In the elderly, the frequency and severity of adverse events associated with the use of cyclobenzaprine, with or without concomitant medications, is increased. In elderly patients, cyclobenzaprine hydrochloride should be initiated with a 5 mg dose and titrated slowly upward.
Patients should be cautioned about the risk of serotonin syndrome with the concomitant use of Cyclobenzaprine Hydrochloride and other drugs, such as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. Patients should be advised of the signs and symptoms of serotonin syndrome, and be instructed to seek medical care immediately if they experience these symptoms (see WARNINGS, and see PRECAUTIONS, Drug Interactions)
Drug Interactions
Cyclobenzaprine HCl may have life-threatening interactions with MAO inhibitors. (See CONTRAINDICATIONS.) Postmarketing cases of serotonin syndrome have been reported during combined use of Cyclobenzaprine Hydrochloride and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. If concomitant treatment with Cyclobenzaprine Hydrochloride and other serotonergic drugs is clinically warranted, careful observation is advised, particularly during treatment initiation or dose increases (see WARNINGS).
Cyclobenzaprine HCl may enhance the effects of alcohol, barbiturates, and other CNS depressants.
Tricyclic antidepressants may block the antihypertensive action of guanethidine and similarly acting compounds.
Tricyclic antidepressants may enhance the seizure risk in patients taking tramadol. †
________________________________________
† ULTRAM® (tramadol hydrochloride tablets, Ortho-McNeil Pharmaceutical)
† ULTRACET® (tramadol hydrochloride and acetaminophen tablets, Ortho-McNeil Pharmaceutical)
Carcinogenesis, Mutagenesis, Impairment of Fertility
In rats treated with cyclobenzaprine HCl for up to 67 weeks at doses of approximately 5 to 40 times the maximum recommended human dose, pale, sometimes enlarged, livers were noted and there was a dose-related hepatocyte vacuolation with lipidosis. In the higher dose groups this microscopic change was seen after 26 weeks and even earlier in rats which died prior to 26 weeks; at lower doses, the change was not seen until after 26 weeks.
Cyclobenzaprine did not affect the onset, incidence or distribution of neoplasia in an 81-week study in the mouse or in a 105-week study in the rat.
At oral doses of up to 10 times the human dose, cyclobenzaprine did not adversely affect the reproductive performance or fertility of male or female rats. Cyclobenzaprine did not demonstrate mutagenic activity in the male mouse at dose levels of up to 20 times the human dose.
Pregnancy
Pregnancy Category B: Reproduction studies have been performed in rats, mice and rabbits at doses up to 20 times the human dose, and have revealed no evidence of impaired fertility or harm to the fetus due to cyclobenzaprine HCl. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because cyclobenzaprine is closely related to the tricyclic antidepressants, some of which are known to be excreted in human milk, caution should be exercised when cyclobenzaprine HCl is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of cyclobenzaprine hydrochloride in pediatric patients below 15 years of age have not been established.
Use in the Elderly
The plasma concentration of cyclobenzaprine is increased in the elderly (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Elderly). The elderly may also be more at risk for CNS adverse events such as hallucinations and confusion, cardiac events resulting in falls or other sequelae, drug-drug and drug-disease interactions. For these reasons, in the elderly, cyclobenzaprine should be used only if clearly needed. In such patients cyclobenzaprine HCl should be initiated with a 5 mg dose and titrated slowly upward.
-
ADVERSE REACTIONS
Incidence of most common adverse reactions in the 2 double-blind‡, placebo-controlled 5 mg studies (incidence of > 3% on cyclobenzaprine HCl tablets, 5 mg):
Cyclobenzaprine HCl
Tablets, 5 mg
N=464Cyclobenzaprine HCl
Tablets, 10 mg
N=249Placebo
N=469Drowsiness 29% 38% 10% Dry Mouth 21% 32% 7% Fatigue 6% 6% 3% Headache 5% 5% 8% Adverse reactions which were reported in 1% to 3% of the patients were: abdominal pain, acid regurgitation, constipation, diarrhea, dizziness, nausea, irritability, mental acuity decreased, nervousness, upper respiratory infection, and pharyngitis.
The following list of adverse reactions is based on the experience in 473 patients treated with cyclobenzaprine HCl tablets, 10 mg in additional controlled clinical studies, 7607 patients in the postmarketing surveillance program, and reports received since the drug was marketed. The overall incidence of adverse reactions among patients in the surveillance program was less than the incidence in the controlled clinical studies.
The adverse reactions reported most frequently with cyclobenzaprine HCl were drowsiness, dry mouth and dizziness. The incidence of these common adverse reactions was lower in the surveillance program than in the controlled clinical studies:
‡ Note: Cyclobenzaprine HCl tablets, 10 mg data are from one clinical trial. Cyclobenzaprine HCl tablets, 5 mg and placebo data are from two studies.
Clinical Studies With
Cyclobenzaprine HCl
Tablets, 10 mgSurveillance Program
With Cyclobenzaprine HCl
Tablets, 10 mgDrowsiness 39% 16% Dry Mouth 27% 7% Dizziness 11% 3% Among the less frequent adverse reactions, there was no appreciable difference in incidence in controlled clinical studies or in the surveillance program. Adverse reactions which were reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion.
The following adverse reactions have been reported in post-marketing experience or with an incidence of less than 1% of patients in clinical trials with the 10 mg tablet:
Body as a Whole: Syncope; malaise.
Cardiovascular: Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension.
Digestive: Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice and cholestasis.
Hypersensitivity: Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash.
Musculoskeletal: Local weakness.
Nervous System and Psychiatric: Seizures, ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis, abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia.
Skin: Sweating.
Special Senses: Ageusia; tinnitus.
Urogenital: Urinary frequency and/or retention.
Causal Relationship Unknown
Other reactions, reported rarely for cyclobenzaprine HCl under circumstances where a causal relationship could not be established or reported for other tricyclic drugs, are listed to serve as alerting information to physicians:
Body as a whole: Chest pain; edema.
Cardiovascular: Hypertension; myocardial infarction; heart block; stroke.
Digestive: Paralytic ileus, tongue discoloration; stomatitis; parotid swelling.
Endocrine: Inappropriate ADH syndrome.
Hematic and Lymphatic: Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia.
Metabolic, Nutritional and Immune: Elevation and lowering of blood sugar levels; weight gain or loss.
Musculoskeletal: Myalgia.
Nervous System and Psychiatric: Decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell's palsy; alteration in EEG patterns; extrapyramidal symptoms.
Respiratory: Dyspnea.
Skin: Photosensitization; alopecia.
Urogenital: Impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea.
-
DRUG ABUSE AND DEPENDENCE
Pharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when cyclobenzaprine HCI is administered, even though they have not been reported to occur with this drug. Abrupt cessation of treatment after prolonged administration rarely may produce nausea, headache, and malaise. These are not indicative of addiction.
-
OVERDOSAGE
Although rare, deaths may occur from overdosage with cyclobenzaprine HCl. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose. As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity may develop rapidly after cyclobenzaprine overdose; therefore, hospital monitoring is required as soon as possible. The acute oral LD50 of cyclobenzaprine HCl is approximately 338 and 425 mg/kg in mice and rats, respectively.
Manifestations
The most common effects associated with cyclobenzaprine overdose are drowsiness and tachycardia. Less frequent manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations. Rare but potentially critical manifestations of overdose are cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome.
Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of cyclobenzaprine toxicity.
Other potential effects of overdosage include any of the symptoms listed under ADVERSE REACTIONS.
Management
General
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
In order to protect against the rare but potentially critical manifestations described above, obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. Observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. Monitoring of plasma drug levels should not guide management of the patient. Dialysis is probably of no value because of low plasma concentrations of the drug.
Gastrointestinal Decontamination
All patients suspected of an overdose with cyclobenzaprine HCl should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage and emesis is contraindicated.
Cardiovascular
A maximal limb-lead QRS duration of ≥0.10 seconds may be the best indication of the severity of the overdose. Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed), should be instituted for patients with dysrhythmias and/or QRS widening. A pH >7.60 or a pCO2<20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines or, if these are ineffective, other anticonvulsants (e.g. phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in close consultation with a poison control center.
-
DOSAGE AND ADMINISTRATION
For most patients, the recommended dose of cyclobenzaprine HCl tablets USP is 5 mg three times a day. Based on individual patient response, the dose may be increased to 10 mg three times a day. Use of cyclobenzaprine HCl tablets USP for periods longer than two or three weeks is not recommended. (see INDICATIONS AND USAGE).
Less frequent dosing should be considered for hepatically impaired or elderly patients (see PRECAUTIONS, Impaired Hepatic Function, and Use in the Elderly).
-
HOW SUPPLIED
Cyclobenzaprine HCl tablets USP are available in 5 mg and 10 mg dosage strengths.
The 5 mg tablets are beige colored, film coated, round, biconvex tablets debossed with ‘ IG’ on one side and “ 282” on other. The 10 mg tablets are yellow colored, film coated, round, biconvex tablets debossed with ‘ IG’ on one side and “ 283” on other. These are supplied as follows:NDC: 50268-190-15 (10 tablets per card, 5 cards per carton).
The 10 mg tablets are yellow colored, film coated, round, biconvex tablets debossed with ‘IG’ on one side and “283” on other.
Dispensed in Unit Dose Package. For Institutional Use Only.
STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].Rx Only
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478Mfg. Rev. 07/16
AV Rev. 08/16 (P)
AvPAK
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 50268-190-15
CyclobenzaprineHydrochloride
Tablets USP
5 mg
Rx Only
50 Tablets (5 X 10) Unite Dose
5026819015
NDC 50268-190-15
CyclobenzaprineHydrochloride
Tablets USP
5 mg
Rx Only
50 Tablets (5 X 10) Unite Dose
5026819015USUAL ADULT DOSAGE:
See accompanying product information.
Store at 20º to 25ºC (68º to 77ºF)[See USP Controlled Room Temperature].
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Manufactured for:
AvKARE Inc.
Pulaski, TN 38478AvPAK TM
A PRODUCT OF AvKARE
Mfg. Rev. 11/15 AV Rev. 01/17 (P)
-
INGREDIENTS AND APPEARANCE
CYCLOBENZAPRINE HYDROCHLORIDE
cyclobenzaprine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50268-190(NDC:69097-845) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOBENZAPRINE HYDROCHLORIDE (UNII: 0VE05JYS2P) (CYCLOBENZAPRINE - UNII:69O5WQQ5TI) CYCLOBENZAPRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color yellow (Beige) Score no score Shape ROUND Size 6mm Flavor Imprint Code IG;282 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50268-190-15 50 in 1 BOX 06/10/2014 1 NDC: 50268-190-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090478 06/10/2014 Labeler - AvPAK (832926666)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.