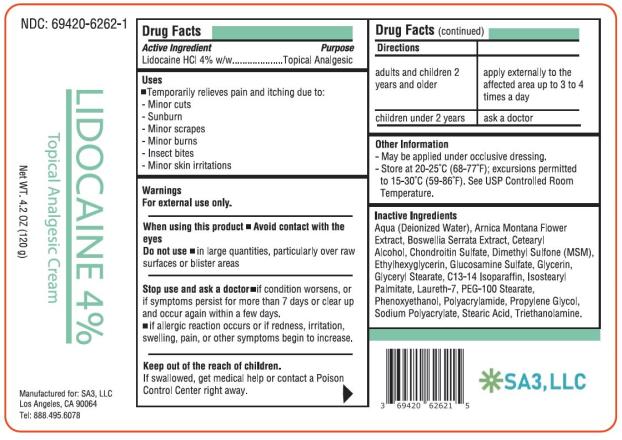
LIDOCAINE 4 PERCENT- lidocaine cream
Lidocaine 4 Percent by
Drug Labeling and Warnings
Lidocaine 4 Percent by is a Otc medication manufactured, distributed, or labeled by SA3, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
LIDOCAINE - Lidocaine HCl 4% Cream
SA3, LLCDisclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lidocaine HCl 4% Topical Analgesic Cream
Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
-
Avoid contact with the eyes
- Do not use in large quantities, particularly over raw surfaces or blistered areas
-
Avoid contact with the eyes
- Directions
- Other information
-
Inactive ingredients
Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cetearyl Alcohol, Chondroitin Sulfate, Dimethyl Sulfone (MSM), Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Stearate, C13-14 Isoparaffin, Isostearyl Palmitate, Laureth-7, PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Propylene Glycol, Sodium Polyacrylate, Stearic Acid, Triethanolamine.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE 4 PERCENT
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69420-6262 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) LAURETH-7 (UNII: Z95S6G8201) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69420-6262-1 120 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2022 Labeler - SA3, LLC (079627454)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.