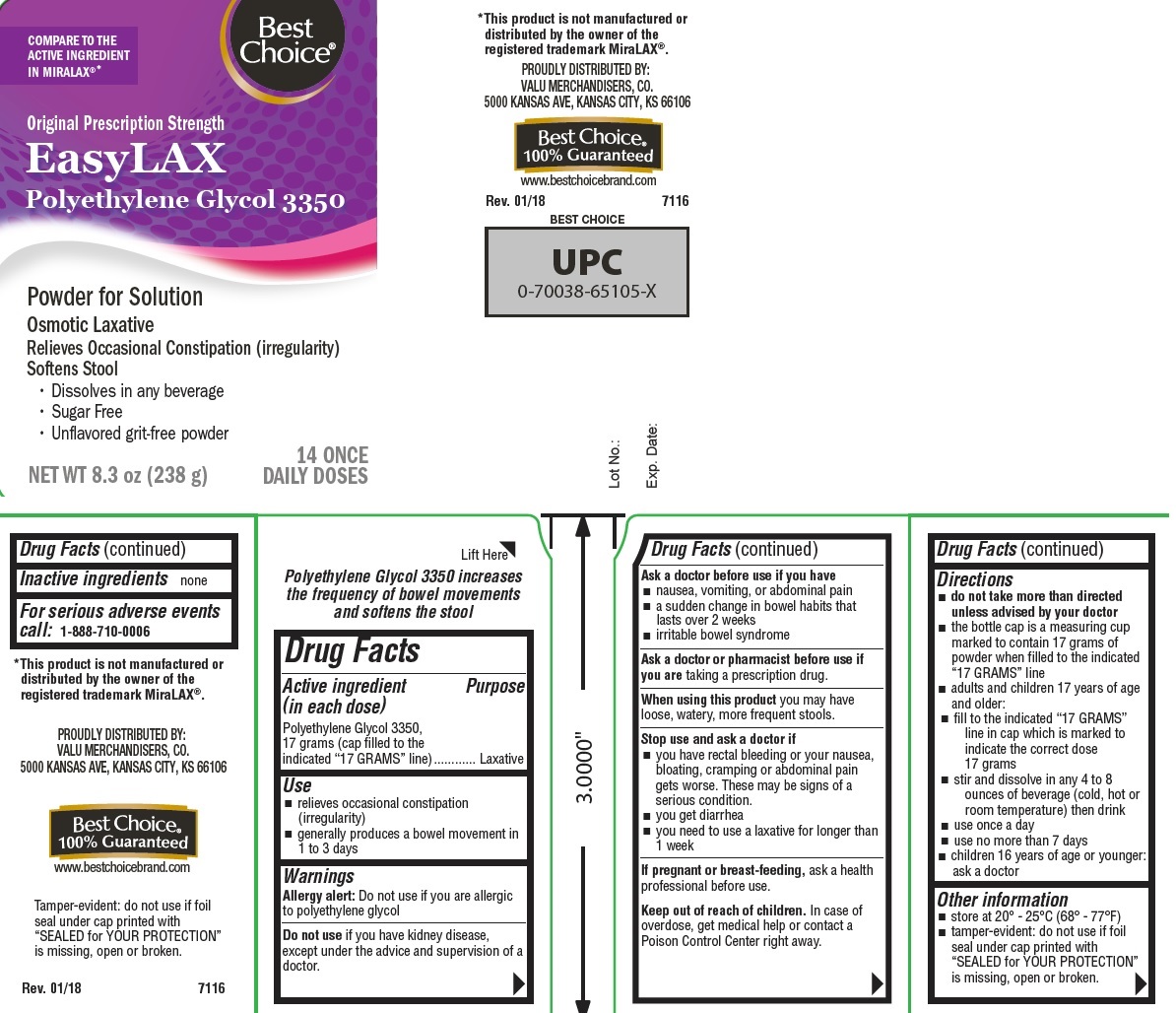
EASYLAX- polyethylene glycol 3350 powder, for solution
EasyLAX by
Drug Labeling and Warnings
EasyLAX by is a Otc medication manufactured, distributed, or labeled by VALU MERCHANDISERS COMPANY, Geri-Care Pharmaceutical Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each dose)
- Purpose
- Use
-
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol
Do not use if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Ask a doctor or pharmacist before use if you are taking a prescription drug
When using this product you may have loose, watery, more frequent stools
Stop use and ask a doctor if
- you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
- you get diarrhea
- you need to use a laxative for longer than 1 week
If pregnant or breast feeding, ask a health professional before use.
-
Directions
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cup marked to contain 17 grams of powder when filled to the indicated “17 GRAMS” line
- adults and children 17 years of age and older:
- fill to indicated “17 GRAMS” line in cap which is marked to indicate the correct dose 17 grams
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use no more than 7 days
- children 16 years of age or younger: ask a doctor
- Other information
- Inactive ingredients
- Package label
-
INGREDIENTS AND APPEARANCE
EASYLAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63941-489 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63941-489-14 238 g in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090812 01/01/2018 Labeler - VALU MERCHANDISERS COMPANY (868703513) Registrant - Geri-Care Pharmaceutical Corp (611196254)
Trademark Results [EasyLAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EASYLAX 77047210 not registered Dead/Abandoned |
Alva International LLC 2006-11-17 |
 EASYLAX 76719358 5171721 Live/Registered |
P&L DEVELOPMENT, LLC 2016-05-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.