DEXMEDESED- dexmedetomidine hydrochloride injection, solution
Dexmedesed by
Drug Labeling and Warnings
Dexmedesed by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION: Dexmedesed (dexmedetomidine hydrochloride) is a synthetic alpha2-adrenoreceptor agonist with sedative and analgesic properties. The chemical name is (+)-4-[1-(2,3-dimethylphenyl) ethyl]-1H-imidazole monohydrochloride. It is a white, or almost white, crystalline, water soluble substance having a molecular weight of 236.7.
The molecular formula is C13 H16 N2 ∙ HCl and the structural formula is:
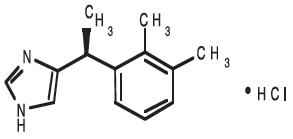
Each mL of Dexmedesed contains 0.5 mg dexmedetomidine hydrochloride, 1.6 mg methylparaben (NF), 0.2 mg propylparaben (NF), 9.0 mg sodium chloride (USP), and water for injection (USP), q.s.
- VETERINARY INDICATIONS
-
DOSAGE AND ADMINISTRATION:
Dogs: Sedation and Analgesia: 500 mcg/m2 intramuscularly (IM) or 375 mcg/m2 intravenously (IV).
Preanesthesia: 125 or 375 mcg/m2 IM.
The choice of preanesthetic dose depends on the duration and severity of the procedure, as well as the anesthetic regime. The following two tables may be used to determine the correct dexmedetomidine hydrochloride dosage. Note that the mcg/kg dosage decreases as body weight increases. For example, dogs weighing 2 kg are dosed at 28.1 mcg/kg dexmedetomidine hydrochloride IV, compared to dogs weighing 80 kg that are dosed at 8.7 mcg/kg. Due to the small volume of administration, accurate dosing is not possible in dogs weighing less than 2 kg (4.4 lb).
Table 1: CANINE SEDATION/ANALGESIA DOSE TABLE: Intravenous (IV) and intramuscular (IM) dosing on the basis of body weight Dexmedesed 0.5 mg/mL Sedation/analgesia in dogs Dog Weight Dexmedetomidine hydrochloride
375 mcg/m2 IVDexmedetomidine hydrochloride
500 mcg/m2 IMlbs kg mcg/kg mL mcg/kg mL 4.4-7 2-3 28.1 0.12 40 0.15 7.1-9 3.1-4 25 0.15 35 0.2 9.1-11 4.1-5 23 0.2 30 0.3 11.1-22 5.1-10 19.6 0.29 25 0.4 22.1-29 10.1-13 16.8 0.38 23 0.5 29.1-33 13.1-15 15.7 0.44 21 0.6 33.1-44 15.1-20 14.6 0.51 20 0.7 44.1-55 20.1-25 13.4 0.6 18 0.8 55.1-66 25.1-30 12.6 0.69 17 0.9 66.1-73 30.1-33 12 0.75 16 1 73.1-81 33.1-37 11.6 0.81 15 1.1 81.1-99 37.1-45 11 0.9 14.5 1.2 99.1-110 45.1-50 10.5 0.99 14 1.3 110.1-121 50.1-55 10.1 1.06 13.5 1.4 121.1-132 55.1-60 9.8 1.13 13 1.5 132.1-143 60.1-65 9.5 1.19 12.8 1.6 143.1-154 65.1-70 9.3 1.26 12.5 1.7 154.1-176 70.1-80 9 1.35 12.3 1.8 >176 >80 8.7 1.42 12 1.9 Table 2: CANINE PREANESTHESIA DOSE TABLE: Intramuscular (IM) dosing on the basis of body weight Dexmedesed 0.5 mg/mL Preanesthesia in dogs Dog Weight Dexmedetomidine hydrochloride
125 mcg/m2 IMDexmedetomidine hydrochloride
375 mcg/m2 IMlbs kg mcg/kg mL mcg/kg mL 4.4-7 2-3 9.4 0.04 28.1 0.12 7.1-9 3.1-4 8.3 0.05 25 0.15 9.1-11 4.1-5 7.7 0.07 23 0.2 11.1-22 5.1-10 6.5 0.1 19.6 0.29 22.1-29 10.1-13 5.6 0.13 16.8 0.38 29.1-33 13.1-15 5.2 0.15 15.7 0.44 33.1-44 15.1-20 4.9 0.17 14.6 0.51 44.1-55 20.1-25 4.5 0.2 13.4 0.6 55.1-66 25.1-30 4.2 0.23 12.6 0.69 66.1-73 30.1-33 4 0.25 12 0.75 73.1-81 33.1-37 3.9 0.27 11.6 0.81 81.1-99 37.1-45 3.7 0.3 11 0.9 99.1-110 45.1-50 3.5 0.33 10.5 0.99 110.1-121 50.1-55 3.4 0.35 10.1 1.06 121.1-132 55.1-60 3.3 0.38 9.8 1.13 132.1-143 60.1-65 3.2 0.4 9.5 1.19 143.1-154 65.1-70 3.1 0.42 9.3 1.26 154.1-176 70.1-80 3 0.45 9 1.35 >176 >80 2.9 0.47 8.7 1.42 The use of dexmedetomidine hydrochloride as a preanesthetic markedly reduces anesthetic requirements in dogs. Injectable induction drug requirements for intubation will be reduced between 30% and 60%, depending on the choice of anesthetic and the dexmedetomidine hydrochloride preanesthetic dose. The concentration of inhalation maintenance anesthetic will be reduced between 40% and 60%, depending on the dose of dexmedetomidine hydrochloride. The anesthetic dose should always be titrated against the response of the patient. The choice of anesthetic is left to the discretion of the veterinarian.
Cats: Sedation, Analgesia and Preanesthesia: 40 mcg/kg intramuscularly (IM).
This dose can also be used as a preanesthetic and has been shown to markedly reduce anesthetic requirements in cats. Injectable anesthetic drug requirements for intubation were reduced up to 49%, depending on the choice of induction drug. The concentration of inhalation maintenance anesthetic was reduced between 35% and 44%, depending on the choice of induction drug. The anesthetic dose should always be titrated against the response of the patient.
The following table may be used to determine the correct dexmedetomidine hydrochloride dosage for cats based on body weight.
Table 3: FELINE DOSE TABLE: Intramuscular (IM) dosing on the basis of body weight in cats Dexmedesed 0.5 mg/mL Sedation/analgesia and preanesthesia in cats Cat Weight Dexmedetomidine hydrochloride
40 mcg/kg IMlbs kg mcg/kg mL 2-4 1-2 40 0.1 4.1-7 2.1-3 40 0.2 7.1-9 3.1-4 40 0.3 9.1-13 4.1-6 40 0.4 13.1-15 6.1-7 40 0.5 15.1-18 7.1-8 40 0.6 18.1-22 8.1-10 40 0.7 It is recommended that dogs and cats be fasted for 12 hours before treatment with Dexmedesed. An eye lubricant should be applied to cats to prevent corneal desiccation that may result from a reduction in the blink reflex. Following injection of Dexmedesed, the animal should be allowed to rest quietly for 15 minutes; sedation and analgesia occur within 5 to 15 minutes, with peak effects at 30 minutes after dexmedetomidine hydrochloride.
-
CONTRAINDICATIONS
CONTRAINDICATIONS: Do not use Dexmedesed in dogs or cats with cardiovascular disease, respiratory disorders, liver or kidney diseases, or in conditions of shock, severe debilitation, or stress due to extreme heat, cold or fatigue.
As with all alpha2-adrenoceptor agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation), exists.
-
WARNINGS:
Human safety: Not for human use. Keep out of reach of children.
Dexmedetomidine hydrochloride can be absorbed following direct exposure to skin, eyes, or mouth, and may cause irritation. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Appropriate precautions should be taken while handling and using filled syringes. Accidental topical (including ocular) exposure, oral exposure, or exposure by injection could cause adverse reactions, including sedation, hypotension, and bradycardia. Seek medical attention immediately. Users with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid any exposure to this product.
Caution should be exercised when handling sedated animals. Handling or any other sudden stimuli, including noise, may cause a defense reaction in an animal that appears to be heavily sedated.
The material safety data sheet (MSDS) contains more detailed occupational safety information. To report adverse reactions in users or to obtain a copy of the MSDS for this product call (866) 933-2472.
Note to physician: This product contains an alpha2-adrenergic agonist.
Animal safety: Dexmedetomidine hydrochloride should not be administered in the presence of preexisting hypotension, hypoxia, or bradycardia. Due to the pronounced cardiovascular effects of dexmedetomidine hydrochloride, only clinically healthy dogs and cats (ASA classes I and II) should be treated. Animals should be frequently monitored for cardiovascular function and body temperature during sedation or anesthesia. Dexmedetomidine hydrochloride sedation is not recommended for cats with respiratory disease.
The use of dexmedetomidine hydrochloride as a preanesthetic in dogs and cats significantly reduces the amount of induction and maintenance anesthetic requirements. Careful patient monitoring during anesthetic induction and maintenance is necessary to avoid anesthetic overdose.
-
PRECAUTIONS
PRECAUTIONS: Apnea may occur with dexmedetomidine hydrochloride use. In the event of apnea, additional oxygen should be supplied. Administration of atipamezole to dogs is warranted when apnea is accompanied by bradycardia and cyanotic mucous membranes.
Adverse reaction reports for dexmedetomidine hydrochloride in cats include rare events of severe dyspnea and respiratory crackles diagnosed as acute pulmonary edema. Dyspnea due to the delayed onset of pulmonary edema could develop in rare instances up to three days after dexmedetomidine hydrochloride administration. Some of these acute and delayed pulmonary edema cases have resulted in death although this was not observed in the feline clinical field studies with dexmedetomidine hydrochloride.
In dogs, intramuscular atipamezole may be routinely used to rapidly reverse the effects of dexmedetomidine hydrochloride.
Since analgesic as well as sedative effects will be reversed, pain management may need to be addressed.
In cats, atipamezole has not been evaluated as a routine dexmedetomidine hydrochloride reversal agent. In cats, cases of dyspnea following atipamezole administration have been reported.
Dexmedetomidine hydrochloride has not been evaluated in the presence of other preanesthetics in cats. Although not observed in the feline field studies, death has been reported in cats receiving dexmedetomidine hydrochloride in conjunction with ketamine and butorphanol.
Analgesia resulting from preanesthetic dexmedetomidine hydrochloride may not provide adequate pain control during the postoperative or postprocedural period. Additional pain management should be addressed as needed.
Following administration of dexmedetomidine hydrochloride, a decrease in body temperature is likely to occur unless externally maintained. Once established, hypothermia may persist longer than sedation and analgesia. To prevent hypothermia, treated animals should be kept warm and at a constant temperature during the procedure, and until full recovery.
Nervous or excited animals with high levels of endogenous catecholamines may exhibit a reduced pharmacological response to alpha2-adrenoceptor agonists like dexmedetomidine hydrochloride (ineffectiveness). In agitated animals, the onset of sedative/analgesic effects could be slowed, or the depth and duration of effects could be diminished or nonexistent. Therefore, allow dogs and cats to rest quietly for 10 to 15 minutes after injection. Repeat dosing has not been evaluated.
Administration of anticholinergic agents in dogs or cats at the same time or after dexmedetomidine hydrochloride could lead to adverse cardiovascular effects (secondary tachycardia, prolonged hypertension, and cardiac arrhythmias1,2,3). However, an anticholinergic drug may be administered to dogs at least 10 minutes before dexmedetomidine hydrochloride for the prevention of the dexmedetomidine hydrochlorideinduced reduction in heart rate. Therefore, the routine use of anticholinergics simultaneously with, or after dexmedetomidine hydrochloride in dogs or cats, is not recommended (see ANIMAL SAFETY).
Spontaneous muscle contractions (twitching) can be expected in some dogs sedated with dexmedetomidine hydrochloride. Dexmedetomidine hydrochloride has been evaluated only in fasted dogs; therefore, its effects on fed dogs (for example, the occurrence of vomiting) have not been characterized. In cats, there is a high frequency of vomition whether fed or fasted; therefore, fasting is recommended to reduce stomach contents.
Dexmedetomidine hydrochloride has not been evaluated in dogs younger than 16 weeks of age, in cats younger than 12 weeks of age, or in geriatric dogs and cats.
Dexmedetomidine hydrochloride has not been evaluated for use in breeding, pregnant, or lactating dogs or cats.
-
ADVERSE REACTIONS:
Canine sedation/analgesia field study: In the field study safety analysis, 106 dogs received dexmedetomidine hydrochloride and 107 received medetomidine. Dogs ranged from 16 weeks to 16 years of age, representing 49 breeds.
Table 4 shows the number of dogs displaying each clinical observation (some dogs experienced more than one adverse reaction). The occurrence of ausculted unidentified arrhythmias (some at multiple time points) decreased following the administration of atipamezole.
Canine preanesthesia field study: The preanesthesia field study safety analysis included 192 dogs, between 5 months and 15 years of age, representing 43 breeds enrolled for elective procedures conducted under general anesthesia. Table 5 shows the number of dogs within a treatment group that showed each clinical sign (dogs may have experienced more than one adverse reaction).
Other clinical signs observed in dogs treated with dexmedetomidine hydrochloride include decreased respiratory rate and hypothermia.
Feline sedation/analgesia field study: The field study safety analysis included 242 cats (122 received dexmedetomidine hydrochloride; 120 received xylazine), 6 months to 17 years of age, and representing 19 breeds. Table 6 shows the number of cats reported with an adverse reaction (cats may have experienced more than one adverse reaction).
The most frequently observed adverse reaction was vomiting in both fasted and fed cats. Other infrequent clinical signs observed in cats treated with dexmedetomidine hydrochloride included fatigue, anorexia, cystitis, and peripheral vascular disorder.
One incidence of dyspnea was reported, 43 minutes after dexmedetomidine hydrochloride administration during an oral examination/dental procedure. Prior to dexmedetomidine hydrochloride, the cat was free of clinical signs, but had a history of asthma and respiratory infection. The cat responded successfully to treatment.
Feline preanesthesia field study: The field study safety analysis included 184 cats (116 received dexmedetomidine hydrochloride; 68 received saline), 12 weeks to 16 years of age, and representing 11 breeds. Table 7 shows the number of cats reported with an adverse reaction (cats may have experienced more than one adverse reaction).
One case of apnea was reported in a cat that received ketamine as the induction agent. This cat required artificial ventilation from the start of the procedure until 30 minutes into recovery when the cat began to breathe on its own. The cat recovered without further problems.
To report suspected adverse events, for technical assistance or to obtain a copy of the MSDS, contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Table 4: Adverse reactions during the canine sedation/analgesia field study Dexmedetomidine hydrochloride
Total n=106Medetomidine
Total n=107Ausculted unidentified arrhythmias 19 20 Severe bradycardia requiring treatment 1 1 Apnea requiring treatment 1 0 Slow onset of sedation (exceeding 30 minutes) 1 1 Ineffectiveness (dog standing throughout the study) 3 2 Severe hypothermia requiring treatment 2 0 Prolonged recovery 1 4 Table 5: Adverse reactions during the canine preanesthesia field study Treatment Groups Induction Anesthetic: Propofol Barbiturate Preanesthetic dose: 0 mcg/m2
n=32125 mcg/m2
n=32375 mcg/m2
n=320 mcg/m2
n=32125 mcg/m2
n=32375 mcg/m2
n=32Emesis 4 7 4 2 3 6 Ventricular premature contractions 0 2 0 4 1 0 Diarrhea 1 0 0 3 1 1 Self trauma 0 2 1 2 1 0 Severe bradycardia 0 0 1 0 0 1 Tachycardia 0 0 0 1 1 0 Urinary incontinence 0 0 0 0 0 1 Table 6: Adverse reactions during the feline sedation/analgesia field study Dexmedetomidine hydrochloride
n=122Xylazine
n=120Vomiting 70 82 Urinary incontinence 6 11 Hypersalivation 4 5 Involuntary defecation 4 1 Hypothermia 2 1 Diarrhea 2 0 Arrhythmia 1 2 Corneal ulcer 1 0 Cyanosis 1 0 Dyspnea 1 0 Table 7: Adverse reactions during the feline preanesthesia field study Induction Anesthetic: Ketamine Propofol Preanesthetic Saline
n=37Dexmedetomidine hydrochloride
n=64Saline
n=31Dexmedetomidine hydrochloride
n=52Emesis 2 20 1 12 Pale mucous membranes 11 9 Decreased body temperature 4 Retching 1 1 3 Heart murmur 2 Loose stool 2 Corneal injury 1 Apnea 1 Behavioral change 1 Fluid in endotracheal tube 1 POST APPROVAL EXPERIENCE: The following adverse events were obtained from post-approval adverse drug events reported for dexmedetomidine hydrochloride from 2007-2009. Not all adverse reactions are reported. Some adverse reactions occurred when dexmedetomidine hydrochloride was used alone for sedation; most occurred when dexmedetomidine hydrochloride was used in the presence of anesthetics and/or other preanesthetics.
It is not always possible to reliably estimate the frequency of an adverse event or to establish a causal relationship to the drug, especially when multiple drugs are administered.
The following reported adverse events are listed in decreasing order of frequency:
Dogs: ineffective for sedation, death, bradycardia, cardiac arrest, apnea, convulsions, vomiting, prolonged sedation, elevated temperature, and delayed sedation.
Cats: ineffective for sedation, death, cardiac arrest, vomiting, apnea, prolonged sedation, hypersalivation, hypothermia, bradycardia, cyanotic mucous membranes, sedation too brief, and dyspnea.
- INFORMATION FOR OWNERS/CAREGIVERS
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Dexmedetomidine hydrochloride is a potent non-narcotic alpha2-adrenoceptor agonist which produces sedation and analgesia. These effects are dose dependent in depth and duration. Blood pressure is initially increased due to peripheral vasoconstriction, subsequently dropping to normal or slightly below normal levels. Vasoconstriction may cause mucous membranes to appear pale or mildly cyanotic. This initial vasopressor response is accompanied by a compensatory marked decrease in heart rate mediated by a vagal baroreceptor. The peripheral pulse may feel weak and a transient change in the conductivity of the cardiac muscle may occur, as evidenced by first and second degree atrioventricular blocks. Other arrhythmias may occur. Dexmedetomidine hydrochloride also decreases the respiratory rate and decreases body temperature. The magnitude and duration of the decrease in body temperature is dose dependent. Dexmedetomidine hydrochloride causes depression of gastrointestinal motility due to decrease in smooth muscle activity, increases in blood glucose levels due to inhibition of insulin release, and increases in production of urine. Spontaneous muscle contractions (twitching) can be expected in some dogs sedated with dexmedetomidine hydrochloride. Vomiting in cats has been associated with alpha2-adrenergic agonist central stimulation of the brain4.
-
EFFECTIVENESS:
Canine sedation/analgesia field study: Dexmedetomidine hydrochloride was evaluated in a masked, controlled, multi-site field study, using parallel treatment groups. Effectiveness was evaluated in 200 (of 213) healthy client-owned dogs, ranging in age between 16 weeks and 16 years of age, and in size between 4.8 lbs and 141 lbs (2.2 kg and 64 kg). Dogs admitted to veterinary clinics for various procedures requiring sedation and/or analgesia received either dexmedetomidine hydrochloride or medetomidine once, by IV or IM injection. Procedures included dental care, radiography, minor skin tumor removal, and treatment of otitis.
Sedation and analgesia occurred within 5 minutes after IV dexmedetomidine hydrochloride, and within 15 minutes after IM dexmedetomidine hydrochloride, with peak effects approximately at 15 or 30 minutes, respectively. Effects waned by approximately two hours after IV administration, and by three hours using the IM route. Dexmedetomidine hydrochloride and medetomidine showed comparable clinical effects. Cardiac rhythms were evaluated by auscultation. Bradycardia occurred within 5 to 15 minutes after IV dexmedetomidine hydrochloride or medetomidine, and within 15 to 30 minutes after either drug given IM. Sixty-four dexmedetomidine hydrochloride-treated dogs and 50 medetomidine-treated dogs were observed with bradycardia.
Adverse reactions during the field study included ausculted unidentified arrhythmias, apnea, hypothermia, and ineffectiveness (see ADVERSE REACTIONS).
Eleven dogs received concomitant medication during the field study, including amoxicillin, cephalexin, triamcinolone, methyl-prednisolone acetate, neomycin, nystatin, thiostrepton, acepromazine, atropine, and atipamezole.
The results of this field study demonstrate that dexmedetomidine hydrochloride produces satisfactory levels of sedation and analgesia for clinical examinations and procedures, minor surgical procedures, and minor dental procedures.
Canine preanesthesia field study: The use of dexmedetomidine hydrochloride as a preanesthetic was evaluated in a controlled, multi-site field study, using parallel treatment groups. Effectiveness was evaluated in 192 healthy, client-owned dogs, between 5 months and 15 years of age, weighing 4 to 196 lbs (2 kg to 89 kg). Dogs received IM dexmedetomidine hydrochloride or saline as a preanesthetic to general anesthesia. All dogs were induced by an injectable anesthetic; half of the dogs were maintained with an inhalation anesthetic. Procedures included orchiectomy, ovariohysterectomy, skin surgery, radiography, physical examination, dental procedures, ear cleaning, anal sac treatment, and grooming. Compared to saline controls, dexmedetomidine hydrochloride IM reduced induction drug requirements by 30-36% (at 125 mcg/m2) and by 38-61% (at 375 mcg/m2). Inhalation anesthetic requirements were 40-60% less for dexmedetomidine hydrochloride-preanesthetized dogs. The number of dogs with clinical signs of pain was less for at least 30 minutes after the procedure in dogs treated with 375 mcg/m2 dexmedetomidine hydrochloride, compared to saline controls.
Recovery times were dose dependent, averaging 15-32 minutes to extubation and 71-131 minutes to standing recovery (longer times correspond to higher dexmedetomidine hydrochloride dose). Recovery times also depended on the induction anesthetic. Recovery times following barbiturate induction were longer (30 minutes to extubation and 118 minutes to standing), compared to dogs induced with propofol (23 minutes to extubation and 84 minutes to standing).
Cardiac arrhythmias were monitored by ECG. Dexmedetomidine hydrochloride-treated dogs were more frequently observed with at least one incidence of arrhythmia compared to saline controls. The most commonly observed arrhythmias were bradycardia, 1st and 2nd degree AV block, and sinus arrest. Other less frequently observed arrhythmias included ventricular premature complexes (VPCs), supraventricular premature complexes, 3rd degree AV block, and sinus pause.
Adverse events included bradycardia, tachycardia, VPCs, vomiting, diarrhea, urinary incontinence, and self trauma (see ADVERSE REACTIONS). The results of the preanesthesia field study demonstrate that dexmedetomidine hydrochloride provided anesthetic dose-sparing, sedation, and analgesia during procedures conducted under general anesthesia.
Feline sedation/analgesia field study: Dexmedetomidine hydrochloride was evaluated in a masked, controlled, multiple site field study, using parallel treatment groups. Effectiveness was evaluated in 242 client-owned cats, ranging in age between 6 months and 17 years, and in size between 2.3 and 9.6 kg (5 and 21 lbs). Cats admitted to veterinary clinics for various procedures requiring restraint, sedation, and/or analgesia were randomized to treatment group and given dexmedetomidine hydrochloride (122 cats) or xylazine (120 cats) once by IM injection. Procedures performed using dexmedetomidine hydrochloride included dental care, radiography, minor superficial surgery, otitis treatment, blood or urine sample collection, tattooing, microchip placement, and grooming.
Sedation and analgesia occurred within 5 to 15 minutes and peak effects were observed 30 minutes after dexmedetomidine hydrochloride. The procedure was easily performed in 91% of cats beginning 30 minutes after dexmedetomidine hydrochloride. Sedative and analgesic effects waned by three hours after dexmedetomidine hydrochloride.
Signs of sedation were deeper for cats receiving dexmedetomidine hydrochloride compared to those receiving xylazine. No clinically relevant differences were observed between dexmedetomidine hydrochloride and xylazine with respect to analgesia or physiological variables. Heart rate, respiratory rate, and rectal temperature decreased. Bradycardia was observed within 5 to 15 minutes and heart rates of ≤70 beats/minute were seen in 18% of cats. The most commonly observed arrhythmias assessed with ECG were atrioventricular dissociation and escape rhythms, followed by a few incidences of premature complexes and one incidence of atrioventricular block. Oxygen saturation, mucous membrane color, capillary refill time, pulse character, respiratory depth and pattern, and response of the animal to injection were clinically satisfactory. All cats recovered from changes induced by dexmedetomidine hydrochloride.
Ninety-seven adverse events were reported after dexmedetomidine hydrochloride. The most frequently reported adverse reactions included vomiting (70), urinary incontinence (6), hypersalivation (4), involuntary defecation (4), hypothermia (2), and diarrhea (2) (see ADVERSE REACTIONS). The results of this field study demonstrate that dexmedetomidine hydrochloride produces satisfactory levels of sedation and analgesia for clinical examinations and procedures, minor surgical procedures, and minor dental procedures.
Feline preanesthesia field study: The use of dexmedetomidine hydrochloride as a preanesthetic was evaluated in a masked, controlled, multi-site field study, using parallel treatment groups. Effectiveness was evaluated in 182 healthy, client-owned cats, between 12 weeks and 16 years of age, weighing 2.10 to 18.8 lbs (0.9 kg to 8.5 kg). Preanesthetic/induction drug regimens included saline/ketamine, dexmedetomidine hydrochloride/ketamine, saline/propofol, and dexmedetomidine hydrochloride/propofol. All cats were intubated prior to the procedure. Inhalant anesthesia (isoflurane) was added during longer procedures (>15 minutes) and could be added during shorter procedures if the veterinarian deemed it necessary. Procedures included ovariohysterectomy, orchiectomy, onychectomy, and dental cleaning.
Dexmedetomidine hydrochloride IM administered at 40 mcg/kg prior to induction with ketamine resulted in a significantly higher proportion of cats that were successfully intubated compared to saline (success rates of 89.5% and 10.7%, respectively).
Cats preanesthetized with dexmedetomidine hydrochloride IM required 48.9% less propofol for successful intubation compared to cats that received saline. Inhalant anesthetic requirements were 35-44% less for dexmedetomidine hydrochloride preanesthetized cats. Recovery times following ketamine and propofol induction averaged 36 and 38 minutes to extubation and 161 and 131 minutes to standing, respectively for dexmedetomidine hydrochloride-treated groups.
Dexmedetomidine hydrochloride (followed by ketamine or propofol) resulted in the following ECG abnormalities (in decreasing order of frequency): sinus bradycardia, sinus arrhythmia, 1st degree atrioventricular (AV) block, long QT interval, sinus pauses, ventricular premature depolarizations, 2nd degree AV block, escape beats/rhythms, and supraventricular premature depolarizations. Dexmedetomidine hydrochloride-treated cats had a lower mean heart rate, respiratory rate, and body temperature compared to saline controls continuing through the recovery period.
Sixty-six adverse events were reported after dexmedetomidine hydrochloride. The most frequently reported adverse events were: vomiting (32), pale mucous membranes (20), decreased body temperature (4), and retching (4) (see ADVERSE REACTIONS).
-
ANIMAL SAFETY:
Canine safety study: In the multiple dose safety study, dexmedetomidine hydrochloride was administered at 0, 1, 3 or 5 times (×) the recommended IV and IM doses on 3 consecutive days to a total of 36 healthy, young beagles. Two additional groups were given a 3× dose of dexmedetomidine hydrochloride (IV or IM) followed by three 1× doses of the reversal agent, atipamezole, every 30 minutes. This was repeated for a total of 3 days. No deaths occurred during the study.
1× dose group: At the recommended dose, sedation lasted less than 3 hours. During sedation, muscle twitches occurred intermittently, and decreases in temperature, respiratory rate and heart rate were observed in all animals. A slow pupil response to light was seen transiently about 15 minutes after dosing in one of twelve dogs. Second degree atrioventricular (AV) blocks were observed in one of twelve dogs.
3× dose group: At 3 times the recommended dose, the duration of sedation was between two and eight hours. During sedation, muscle twitches occurred, and temperature, respiratory rate, and heart rate decreased in all dogs. The pupillary light reflex was transiently decreased for up to 90 minutes in four of twelve dogs. Vomiting was seen in two of twelve dogs. One dog experienced first and second degree AV blocks; second degree AV block was observed in three of twelve dogs. Elevated concentrations of alanine aminotransferase (ALT) were observed in one dog, without histological changes to the liver.
5× dose group: At 5 times the recommended dose, the duration of sedation was between four and eight hours. Muscle twitches, decreases in temperature, respiratory rates, and heart rates were seen in all dogs. No pupil response was noted in six of twelve dogs (IV) for up to 1.5 hours; decreased transient pupillary light reflex was seen for up to 60 minutes in two of twelve dogs (IM). Vomiting was seen in one of twelve dogs. First and second degree AV blocks were observed in one of twelve dogs. Elevated concentrations of ALT were observed in 3 of 12 dogs, without histological changes to the liver.
Dexmedetomidine hydrochloride demonstrated dose dependent effects related to its pharmacology when administered IV or IM to healthy dogs at doses up to five times the recommended dose.
Canine safety study with an anticholinergic: In another laboratory safety study, one of three doses of an IM anticholinergic drug or saline was administered 10 minutes before, at the same time, or 15 minutes after 500 mcg/m2 IM dexmedetomidine hydrochloride. The anticholinergic drug was given for the prevention or treatment of dexmedetomidine hydrochloride-induced reduction in heart rate. In a crossover design, 18 dogs were used in a total of 72 trials, to evaluate the safety of dexmedetomidine hydrochloride used with an anticholinergic drug.
Dogs were instrumented for the accumulation of continuous ECG data. The following arrhythmias were recorded during the study (some dogs experienced more than one arrhythmia).
Table 8: Arrhythmias recorded during the canine laboratory safety study* Type of arrhythmia Number of dogs
(of 18)- * Table does not relate arrhythmias to the presence or absence of anticholinergic
Second degree AV block 18 Supraventricular tachycardia (SVT) or SVPCs 16 Ventricular escape beats 16 Ventricular premature contractions 14 Third degree AV block 6 Idioventricular rhythm 1 Paroxysmal VT 1 Ventricular bigeminy; SVPCs; pulse alternans 1 Junctional escape beat 1 The occurrence of arrhythmias was not related to the presence or absence of the anticholinergic drug. Arrhythmias were transient (although frequent over time in some dogs), returning toward baseline levels within 55 minutes after dexmedetomidine hydrochloride. No dogs required treatment related to these arrhythmias, and none of these arrhythmias persisted or adversely affected the overall clinical status of any dog in the study.
Dexmedetomidine hydrochloride without anticholinergic: Without the anticholinergic drug, and in addition to arrhythmias, dexmedetomidine hydrochloride produced clinically relevant sedation accompanied by a statistically significant reduction in heart rate, respiratory rate, cardiac output, pulmonary arterial temperature, and mixed venous oxygen tension. A statistically significant increase in arterial blood pressure, pulmonary capillary wedge pressure, central venous pressure, and systemic vascular resistance was noted. No dogs experienced hypotension. Dexmedetomidine hydrochloride tended to increase pulmonary vascular resistance. Dexmedetomidine hydrochloride alone had no statistically significant effect on mean pulmonary arterial pressure, arterial pH, arterial carbon dioxide tension, and arterial oxygen tension.
Dexmedetomidine hydrochloride plus anticholinergic: Either of the two higher anticholinergic doses was effective in the prevention or treatment of the dexmedetomidine hydrochloride-induced reduction in heart rate. Anticholinergic (higher doses) given after dexmedetomidine hydrochloride caused marked increases in the occurrence of various cardiac arrhythmias, especially second degree AV block. When the higher doses of anticholinergic drug were given at the same time or 15 minutes after dexmedetomidine hydrochloride, large increases in heart rate (p<0.01) and blood pressure (p<0.05) were seen. Increases were dose related; the highest anticholinergic dose elicited more frequent arrhythmias and larger increases in heart rate and blood pressure.
In conclusion, moderate doses of anticholinergic drug given prior to dexmedetomidine hydrochloride performed best for the prevention of dexmedetomidine hydrochloride-induced reduction of heart rate in dogs. The routine use of anticholinergics given simultaneously with, or after dexmedetomidine hydrochloride, is not recommended.
Feline safety study: In a multiple dose safety study, dexmedetomidine hydrochloride, was administered intramuscularly (IM) at 1×, 3×, and 5× (40, 120, and 200 mcg/kg) the recommended dose of 40 mcg/kg on 3 consecutive days to healthy cats, 6 to 8 months old. A control group received the product vehicle as a placebo (0×). No mortality was observed. The depth and duration of sedation was dose dependent, lasting approximately 2 hours in the 1× group, 2 to 4 hours in the 3× group, and greater than 8 hours in the 5× group. The lowest recorded individual heart rate was 60 beats/minute and occurred in the 5× dose group (2 cats). Cardiac arrhythmias characterized by isolated junctional escape complexes with episodes of junctional escape rhythm were observed during periods of low heart rate or following sinus pauses in all dexmedetomidine hydrochloride dose groups. In most cases the arrhythmia was no longer observed after 1 to 2 hours. Atrioventricular block was not observed. Incidences of arrhythmias were not related to dose; however, more cats were affected by cardiac arrhythmias on the third day of treatment, compared to the first two days of the study. The decrease in respiratory rate, but not the duration, was dose dependent. The rectal temperature decreased in all dexmedetomidine hydrochloride-treated groups, with the lowest temperatures in the 5× group at 8 hours on all three days. Two cats vomited (40 and 120 mcg/kg). Corneal opacity was noted in all dexmedetomidine hydrochloride-dose groups, was transient, related to dose and duration of sedation, and was attributed to lack of lubrication with decreased blinking during sedation. Hematology and blood chemistry were unaffected by treatment. Injection site tolerance was good, with mild inflammatory lesions representative of the IM injection procedure. Gross and histological examination of all other tissues did not reveal any abnormalities related to dexmedetomidine hydrochloride administration.
Dexmedetomidine hydrochloride demonstrated dose dependent effects related to its pharmacology when administered IM to healthy cats at doses up to five times the recommended dose.
Feline acute tolerance study: IM dexmedetomidine hydrochloride was administered once at 10× (400 mcg/kg) the recommended dose of 40 mcg/kg to 3 female and 3 male 7 month old cats. No mortality was observed. Sedation was observed within 15 minutes of dosing and lasted for at least 4 hours with full recovery noted between 8 and 24 hours after dosing. Transient observations of corneal dehydration and opacity, miosis, pale skin and gingiva, salivation, and watery ocular discharge were observed in some animals. Vomiting was observed 7 to 11 hours after dosing in all but one animal. Decreases in heart rate accompanied by prolonged PQ and QT intervals were most pronounced 2 to 4 hours after dosing. No atrioventricular (AV) blocks or escape rhythms were noted. In one cat, incidental and reversible premature junctional complexes were seen at 1 and 2 hours after dosing which were considered secondary to bradycardia. Slightly lower respiratory rate and reduced rectal temperature were observed 4 to 8 hours after dosing. Observations had returned to normal by 24 hours after dosing. Mild inflammatory lesions observed histologically at the injection site were representative of the IM injection procedure. No treatment related changes were observed in hematology. Mild elevations in some clinical ALT, AST, and CK, were observed 24 hours after dosing, with a trend towards recovery by 48 hours. Total protein, albumin and globulin levels were slightly lowered in one cat 48 hours after dosing.
-
STORAGE AND HANDLING
STORAGE INFORMATION: Store at controlled room temperature 68-77°F (20-25°C). Protect from freezing. In use shelf life: 28 days at 77°F (25°C).
HOW SUPPLIED: Dexmedesed is supplied in 10-mL, multidose vials containing 0.5 mg of dexmedetomidine hydrochloride per mL. NDC: 17033-005-10.
-
REFERENCES:
- (1) Ko JCH, Fox SMF, Mandsager RE. Effects of preemptive atropine administration on incidence of medetomidine-induced bradycardia in dogs. J Am Vet Med Assoc 2001; 218:52-58.
- (2) Alibhai HIK, Clarke KW, Lee YH, et al. Cardiopulmonary effects of combinations of medetomidine hydrochloride and atropine sulphate in dogs. Vet Rec 1996; 138:11-13.
- (3) Short, CE. Effects of anticholinergic treatment on the cardiac and respiratory systems in dogs sedated with medetomidine. Vet Rec 1991; 129:310-313.
- (4) Hikasa Y, Akiba T, Iino Y et al. Central alpha-adrenoceptor subtypes involved in the emetic pathway in cats. Eur J Pharmacol 1992; 229:241-251
- SPL UNCLASSIFIED SECTION
-
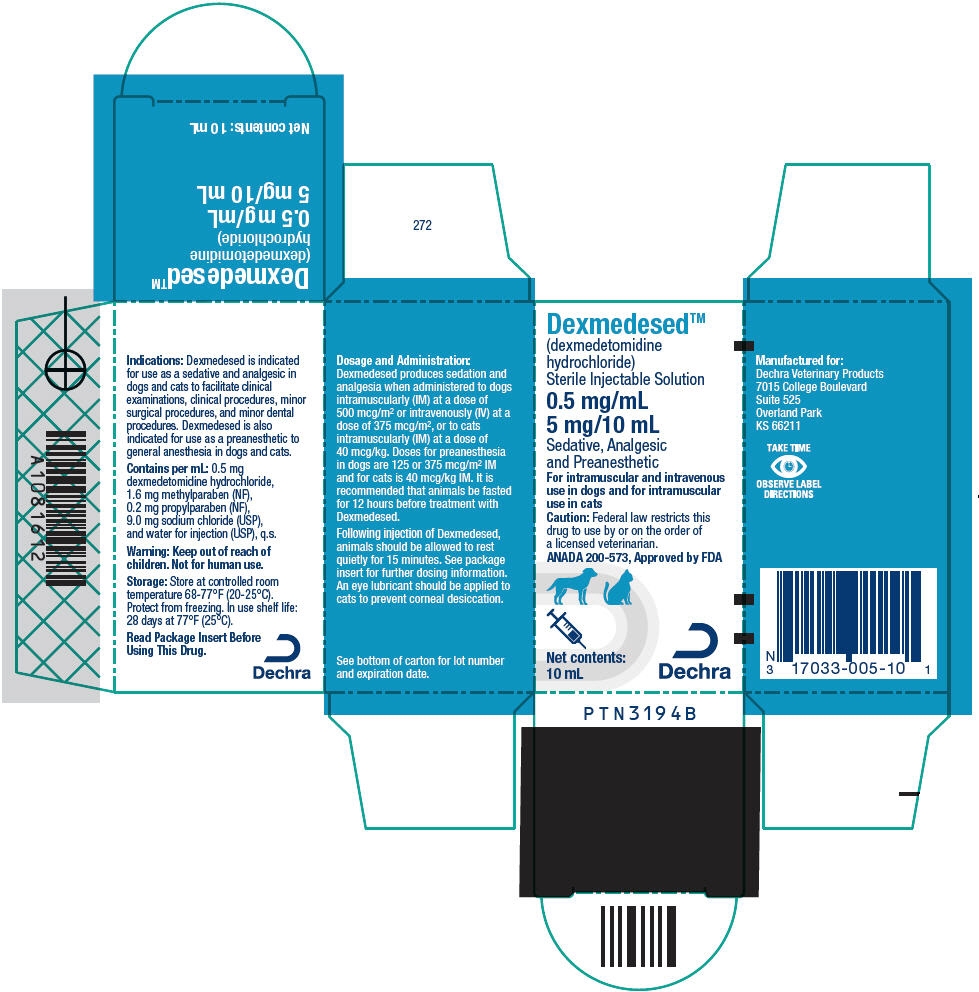
PRINCIPAL DISPLAY PANEL - 5 mg/10 mL Vial Carton
Dexmedesed™
(dexmedetomidine
hydrochloride)
Sterile Injectable Solution0.5 mg/mL
5 mg/10 mLSedative, Analgesic
and PreanestheticFor intramuscular and intravenous
use in dogs and for intramuscular
use in catsCaution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.ANADA 200-573, Approved by FDA
Net contents:
10 mLDechra
-
INGREDIENTS AND APPEARANCE
DEXMEDESED
dexmedetomidine hydrochloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-005 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dexmedetomidine Hydrochloride (UNII: 1018WH7F9I) (Dexmedetomidine - UNII:67VB76HONO) Dexmedetomidine 0.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-005-10 1 in 1 CARTON 1 10 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200573 07/17/2017 Labeler - Dechra Veterinary Products LLC (362142734)
Trademark Results [Dexmedesed]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DEXMEDESED 87251866 5387997 Live/Registered |
Putney, Inc. 2016-11-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
