EXCEDE FOR SWINE STERILE- ceftiofur injection, suspension
Excede for Swine by
Drug Labeling and Warnings
Excede for Swine by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
CAUTION
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits extra-label use of this drug in swine for disease prevention purposes; at unapproved doses, frequencies, durations, or routes of administration; and in unapproved major food producing species/ production classes.
-
DESCRIPTION
EXCEDE FOR SWINE Sterile Suspension 100 mg/mL is a ready-to-use formulation that contains the crystalline free acid of ceftiofur, which is a broad spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria including ß-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal in vitro, resulting from inhibition of cell wall synthesis.
Each mL of this ready-to-use sterile suspension contains ceftiofur crystalline free acid equivalent to 100 mg ceftiofur, in a Caprylic/capric triglyceride and cottonseed oil based suspension.
Figure 1. Structure of ceftiofur crystalline free acid:
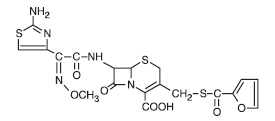
Chemical name of ceftiofur crystalline free acid:
7-[[2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-3-[[(2-furanylcarbonyl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene 2-carboxylic acid
-
INDICATIONS
EXCEDE FOR SWINE Sterile Suspension 100 mg/mL is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis; and for the control of SRD associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis in groups of pigs where SRD has been diagnosed.
-
DOSAGE & ADMINISTRATION
DOSAGE
Administer by intramuscular (IM) injection in the post-auricular region of the neck as a single dosage of 2.27 mg ceftiofur equivalents (CE)/lb (5.0 mg CE/kg) body weight (BW). This is equivalent to 1 mL sterile suspension per 44 lb (20 kg) BW. No more than 2 mL should be injected in a single injection site. Injection volumes in excess of 2 mL per injection site may result in violative residues. Pigs heavier than 88 lb (40 kg) will require more than one injection.
Most animals will respond to treatment within three to five days. If no improvement is observed, the diagnosis should be re-evaluated.
- ADMINISTRATION
- CONTRAINDICATIONS
-
WARNINGS
FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing protective gloves.
Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product.
In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
The material safety data sheet contains more detailed occupational safety information. To report adverse effects in users, to obtain more information or to obtain a material safety data sheet, call 1-888-963-8471.
-
RESIDUE WARNINGS
- A maximum of 2 mL of formulation should be injected at each injection site. Injection volumes in excess of 2 mL per injection site may result in violative residues.
- Following label use as a single treatment, a 14-day pre-slaughter withdrawal period is required.
- Use of dosages in excess of 5.0 mg ceftiofur equivalents (CE)/kg or administration by an unapproved route may result in illegal residues in edible tissues.
-
PRECAUTIONS
The safety of ceftiofur has not been demonstrated for pregnant swine or swine intended for breeding.
Administration of EXCEDE FOR SWINE Sterile Suspension 100 mg/mL as directed may induce a transient reaction at the site of injection and underlying tissues that may result in trim loss of edible tissue at slaughter.
-
ADVERSE REACTIONS
An injection site tolerance study demonstrated that EXCEDE FOR SWINE Sterile Suspension 100 mg/mL is well tolerated in pigs. Half of the injection sites at both 3 and 7 days post-injection were scored as "negative" for irritation and the other half were scored as "slight irritation". All gross observations and measurements of injection sites qualified the sites at 10 days post-injection as "negative" for irritation.
No adverse effects were observed in multi-location field efficacy studies involving more than 1000 pigs.
-
CLINICAL PHARMACOLOGY
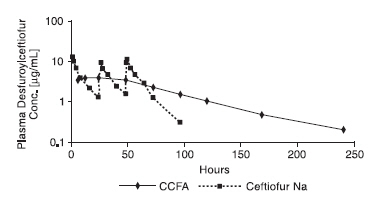
Ceftiofur administered as either ceftiofur sodium (NAXCEL® Sterile Powder), ceftiofur hydrochloride (EXCENEL® RTU Sterile Suspension) or ceftiofur crystalline free acid (EXCEDE FOR SWINE Sterile Suspension 100 mg/mL) is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to swine as ceftiofur crystalline free acid (CCFA) at a single IM dosage of 2.27 mg CE/lb (5.0 mg CE/kg) BW provides concentrations of ceftiofur and desfuroylceftiofurrelated metabolites in plasma that are multiples above the MIC901 for the SRD label pathogens Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis and Streptococcus suis for an extended period of time (see Figure 2 and Tables 1–2).
The average plasma concentrations of ceftiofur- and desfuroylceftiofur-related metabolites for CCFA (EXCEDE FOR SWINE Sterile Suspension 100 mg/mL) after IM administration of 2.27 mg CE/lb (5.0 mg CE/kg) BW and those for ceftiofur sodium (NAXCEL Sterile Powder) after IM administration at 1.36 mg CE/lb (3 mg CE/kg) BW for three consecutive days are presented in Figure 2 below.
Figure 2. Average plasma concentrations of ceftiofur- and desfuroylceftiofur-related metabolites for CCFA (EXCEDE FOR SWINE Sterile Suspension 100 mg/mL) after IM administration of 2.27 mg CE/lb (5.0 mg CE/kg) BW and those for ceftiofur sodium (NAXCEL Sterile Powder) after IM administration at 1.36 mg CE/lb (3 mg CE/kg) BW for three consecutive days
Pharmacokinetic parameters measured after a single IM administration of 2.27 mg CE/lb (5.0 mg CE/kg) BW of EXCEDE FOR SWINE Sterile Suspension 100 mg/mL in the post-auricular region of the neck of swine are presented in the following table (Table 1).
Table 1. Pharmacokinetic parameters in swine after a single IM administration of EXCEDE FOR SWINE Sterile Suspension 100 mg/mL at 2.27 mg CE/lb (5.0 mg CE/kg) BW Pharmacokinetic
ParameterMean Value ± Standard Deviation
(non-compartmental analyses)Cmax = maximum plasma concentration (in μg CE/mL) tmax = the time after injection when Cmax occurs (in hours) AUC 0-LOQ = the area under the plasma concentration vs. time curve from time of injection to the limit of quantitation of the assay (0.15 μg CE/mL) t1/2 = terminal phase biological half-life (in hours) Cmax (μg/mL) 4.17 ± 0.92 tmax (h) 22.0 ± 12.2 AUC 0-LOQ (μg∙h/mL) 373.0 ± 56.1 t1/2 (h) 49.6 ± 11.8 Table 2. Ceftiofur minimum inhibitory concentration (MIC) values* of indicated pathogens isolated from SRD treatment and control field studies conducted in the U.S. Indicated Pathogens Year(s) of Isolation Field Study Number of Isolates MIC50†
(μg/mL)MIC90†
(μg/mL)MIC Range
(μg/mL)- * The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
- † The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
- ‡ No range; all isolates yielded the same value.
Actinobacillus pleuropneumoniae 2000 to 2001 Treatment 5 NA NA ≤0.03 to 0.06 2009 Control 34 0.03 0.06 0.015 to 0.06 Pasteurella multocida 2000 to 2001 Treatment 20 ≤0.03 ≤0.03 ≤0.03‡ 2009 Control 67 ≤0.004 ≤0.004 ≤0.004‡ Streptococcus suis 2000 to 2001 Treatment 30 0.06 0.12 ≤ 0.03 to 0.5 2009 Control 141 0.25 1 0.03 to >2
- 1 Minimum inhibitory concentration for 90% of the isolates
MICROBIOLOGY
Ceftiofur has demonstrated in vitro activity against Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis, four major pathogenic bacteria associated with SRD.
The minimum inhibitory concentrations (MICs) of ceftiofur against indicated SRD pathogens were determined using methods recommended by the Clinical and Laboratory Standards Institute (CLSI) using the M31-A and M31-A3 standards for the SRD treatment (2000-2001) and control (2009) studies, respectively. Isolates from the SRD treatment study were obtained from lung tissue collected from non-treated pigs prior to enrollment and saline-treated pigs that died or were euthanized during the study. Isolates from the SRD control study were obtained from lung tissue from non-treated pigs euthanized prior to enrollment and from saline- and ceftiofur-treated pigs that died or were euthanized during the study. The susceptibility results for the treatment and control studies are presented in Table 2.
Based on pharmacokinetic data from studies of ceftiofur in swine after a single intramuscular injection of 2.27 mg CE/lb (5.0 mg CE/kg) BW, the following interpretive criteria are recommended by CLSI:
Table 3. CLSI-accepted interpretive criteria for ceftiofur against swine respiratory disease pathogens* Pathogens Disk Potency Zone Diameter
(mm)MIC
(μg/mL)Interpretation - * These interpretive criteria should only be used when the CLSI M31-A3 performance standard is used to determine antimicrobial susceptibility to ceftiofur.
Actinobacillus pleuropneumoniae
Pasteurella multocida
Streptococcus suis30 μg ≥21 ≤2.0 (S) Susceptible 18-20 4.0 (I) Intermediate ≤17 ≥8.0 (R) Resistant -
EFFECTIVENESS
The effectiveness of a single dose of 2.27 or 3.18 mg CE/lb BW (5.0 or 7.0 mg CE/kg BW) EXCEDE FOR SWINE Sterile Suspension 100 mg/mL for the treatment of SRD was confirmed in a well-controlled, multi-location field study. A total of 706 pigs with clinical signs of bacterial respiratory disease were enrolled and treated with a placebo injection or EXCEDE FOR SWINE Sterile Suspension 100 mg/mL administered as a single IM injection in the post-auricular region of the neck. Clinical observations were performed on Days 1-7 and rectal temperatures were taken on Days 1, 3, and 6 following treatment (Day 0). Necropsies were performed on all pigs that died during the study and after euthanasia of all remaining study pigs at the end of the 14-day post-enrollment study period. Lung lesions were scored and lungs were submitted for bacterial identification. Mortality rates were numerically lower (but not statistically different) for the EXCEDE FOR SWINE Sterile Suspension 100 mg/mL-treated groups (4.3% for the 5.0 mg CE/kg BW group and 4.2% for the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (6.3%). There was a statistically significant (p<0.05) improvement in clinical cure rates for the EXCEDE FOR SWINE Sterile Suspension 100 mg/mL-treated groups (24.8% for the 5.0 mg CE/kg BW group and 26.4% for the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (17.7%). Lung lesion scores were numerically higher (but not statistically different) for the EXCEDE FOR SWINE Sterile Suspension 100 mg/mL-treated groups (10.4% for both the 5.0 mg CE/kg BW and the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (9.2%). Bacteriological culture of the lungs of study pigs identified the following respiratory pathogens: Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis.
The effectiveness of a single dose of 2.27 CE/lb BW (5.0 mg CE/kg BW) EXCEDE FOR SWINE for the control of SRD was evaluated in a multi-location natural infection field study. At each site, when at least 15% of the study candidates in a pen showed clinical signs of SRD, all pigs in the pen were enrolled and treated with EXCEDE FOR SWINE (n = 346) or saline (n = 347). Responses to treatment were evaluated 7 days post-treatment. Success was defined as a pig that survived to Day 7 and had normal attitude, normal respiration, and a rectal temperature of < 104 °F. The treatment success rate was significantly higher (p = 0.0188) for EXCEDE FOR SWINE-treated pigs (59.6%) compared to the saline-treated pigs (41.4%). Bacteriological culture of the lungs of study pigs identified the following respiratory pathogens: Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis.
-
ANIMAL SAFETY
After parenteral administration, CCFA, ceftiofur sodium, and ceftiofur hydrochloride are metabolized to the same principal metabolite, desfuroylceftiofur. Plasma levels achieved are similar after recommended dosing (Figure 2). Therefore, studies conducted with ceftiofur sodium are adequate to evaluate the systemic safety of CCFA. Results from a five-day tolerance study in normal feeder pigs indicated that ceftiofur sodium produced no overt adverse signs of toxicity and was well tolerated when administered at 57 mg CE/lb (125 mg/kg) BW (more than 25 times the recommended dosage of CCFA) for five consecutive days.
Table 4. Acceptable quality control ranges for ceftiofur against CLSI recommended American Type Culture Collection (ATCC) reference strains Organism Name (ATCC No.) MIC (μg/mL) Zone Diameter, mm
(Disk Content 30 μg)E. coli ATCC 25922 0.25–1.0 26–31 S. aureus ATCC 29213 0.25–1.0 — S. aureus ATCC 25923 — 27–31 P. aeruginosa ATCC 27853 16.0–64.0 14–18 An additional dose toxicity study was conducted to determine the safety margin of ceftiofur in swine. Five barrows and five gilts per group were administered ceftiofur sodium IM at 0, 2.27, 6.81 and 11.36 mg CE/lb (0, 5, 15, 25 mg CE/kg) BW (0, 1, 3 and 5 times the recommended dosage for CCFA) for 15 consecutive days. There were no adverse systemic effects observed, indicating that ceftiofur sodium has a wide margin of safety when administered intramuscularly in feeder pigs.
A separate study evaluated the injection site tissue tolerance of EXCEDE FOR SWINE Sterile Suspension 100 mg/mL in swine when administered intramuscularly as a single injection at the maximum recommended dose volume of 2 mL (approximately 5 mg CE/kg BW) per injection site. Because injection site volumes greater than 2 mL may result in violative residues, only injection volumes of 2 mL were evaluated in this study. EXCEDE FOR SWINE Sterile Suspension 100 mg/mL was injected intramuscularly into each side of the neck of six swine at a dose volume of 2 mL/injection site. Clinical observations were made daily. At 3, 7 and 10 days post-injection, pairs of animals were euthanized and the neck injection sites were dissected for pathological examination (4 injection sites per time point). The injections were well tolerated in all pigs. Clinically, injection site reactions ranged from nondetectable (6 of 12 sites) to a transitory (up to 4 days post-injection) palpable, nonvisible swelling (2 of 12 sites) or a small, visible, reddened nodule at the needle insertion point (4 of 12 sites; 3 of 4 nodules were barely detectable by 3 to 7 days post-injection). There was no clinical evidence of the injections at 10 days post-injection. At necropsy, half of the injection sites at both 3 and 7 days post-injection were scored as "negative" for irritation and the other half were scored as "slight irritation". One animal had a visible lesion described as an area of tan with red speckles present in the deep muscle fascia, less than 6 cm2, at 10 days post-injection; this lesion and the remaining injection sites evaluated at 10 days post-injection were scored as "negative" for irritation.
- STORAGE CONDITIONS
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 mL Vial Label
-
INGREDIENTS AND APPEARANCE
EXCEDE FOR SWINE STERILE
ceftiofur injection, suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-5223 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFTIOFUR (UNII: 83JL932I1C) (CEFTIOFUR - UNII:83JL932I1C) CEFTIOFUR 100 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-5223-1 100 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141235 06/18/2004 Labeler - Zoetis Inc. (828851555)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
