IMJUDO- tremelimumab injection, solution
IMJUDO by
Drug Labeling and Warnings
IMJUDO by is a Prescription medication manufactured, distributed, or labeled by AstraZeneca Pharmaceuticals LP, AstraZeneca PLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IMJUDO safely and effectively. See full prescribing information for IMJUDO.
IMJUDO® (tremelimumab-actl) injection, for intravenous use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
IMJUDO is a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blocking antibody indicated:
- in combination with durvalumab, for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC). (1.1)
- in combination with durvalumab and platinum-based chemotherapy for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) with no sensitizing epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) genomic tumor aberrations. (1.2)
DOSAGE AND ADMINISTRATION
- Administer IMJUDO as an intravenous infusion over 60 minutes after dilution. (2.3)
- uHCC:
Weight 30 kg and more: IMJUDO 300 mg as a single dose in combination with durvalumab 1,500 mg at Cycle 1/Day 1, followed by durvalumab as a single agent every 4 weeks (2.1)
Weight less than 30 kg: IMJUDO 4 mg/kg as a single dose in combination with durvalumab 20 mg/kg at Cycle 1/Day 1, followed by durvalumab as a single agent every 4 weeks (2.1)
- Metastatic NSCLC:
Weight 30 kg and more: 75 mg every 3 weeks in combination with durvalumab 1,500 mg and platinum-based chemotherapy for 4 cycles, and then administer durvalumab 1,500 mg every 4 weeks as a single agent with histology-based pemetrexed therapy every 4 weeks, and a fifth dose of IMJUDO 75 mg in combination with durvalumab dose 6 at week 16 (2.1)
Weight less than 30 kg: 1 mg/kg every 3 weeks in combination with durvalumab 20 mg/kg and platinum-based chemotherapy for 4 cycles, and then administer durvalumab 20 mg/kg every 4 weeks as a single agent with histology-based pemetrexed therapy every 4 weeks, and a fifth dose of IMJUDO 1 mg/kg in combination with durvalumab dose 6 at week 16 (2.1)
- See full Prescribing Information for preparation and administration instructions and dosage modifications for adverse reactions.
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Immune-Mediated Adverse Reactions (5.1)
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions and immune-mediated pancreatitis.
-
-
- o Monitor for early identification and management. Evaluate liver enzymes, creatinine, adrenocorticotropic hormone level and thyroid function at baseline and before each dose.
- o Withhold or permanently discontinue based on severity and type of reaction.
-
- Infusion-Related Reactions: Interrupt, slow the rate of infusion, or permanently discontinue treatment based on the severity of the reaction. (5.2)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (≥ 20%) of patients with uHCC are rash, diarrhea, fatigue, pruritus, musculoskeletal pain, and abdominal pain. Most common laboratory abnormalities (≥ 40%) of patients with uHCC are AST increased, ALT increased, hemoglobin decreased, sodium decreased, bilirubin increased, alkaline phosphatase increased, and lymphocytes decreased. (6.1)
Most common adverse reactions (≥ 20%) of patients with metastatic NSCLC were nausea, fatigue, musculoskeletal pain, decreased appetite, rash, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hepatocellular Carcinoma
1.2 Non-Small Cell Lung Cancer (NSCLC)
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Adverse Reactions
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Infusion-Related Reactions
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hepatocellular Carcinoma (HCC)
14.2 Metastatic NSCLC
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Hepatocellular Carcinoma
IMJUDO, in combination with durvalumab, is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
1.2 Non-Small Cell Lung Cancer (NSCLC)
IMJUDO, in combination with durvalumab and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosages of IMJUDO are presented in Tables 1, 2 and 3.
Administer IMJUDO as an intravenous infusion after dilution as recommended [see Dosage and Administration (2.3)].
IMJUDO in Combination with Durvalumab
Table 1. Recommended dosage of IMJUDO - * Administer IMJUDO prior to durvalumab on the same day.
- † Refer to the Prescribing Information for durvalumab dosing information.
Indication
Recommended IMJUDO Dosage
Duration of Therapy
uHCC
Patients with a body weight of 30 kg and more:
- A single dose of IMJUDO* 300 mg followed by durvalumab† 1,500 mg at Day 1 of Cycle 1;
- Continue durvalumab 1,500 mg as a single agent every 4 weeks
Patients with a body weight of less than 30 kg:
- A single dose of IMJUDO* 4 mg/kg followed by durvalumab 20 mg/kg at Day 1 of Cycle 1;
- Continue durvalumab 20 mg/kg as a single agent every 4 weeks
After Cycle 1 of combination therapy, administer durvalumab as a single agent every 4 weeks until disease progression or unacceptable toxicity
IMJUDO in Combination with Durvalumab and Platinum-Based Chemotherapy
The recommended dosage schedule and regimens for IMJUDO for the treatment of metastatic non-small cell lung cancer (NSCLC) are provided in Tables 2 and 3.
Weigh patients prior to each infusion.
Calculate the appropriate dose using Table 3 below based on the patient’s weight and tumor histology.
Table 2: Recommended Dosage Schedule Week*,† 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 - * continue durvalumab until disease progression or intolerable toxicity.
- † dosing interval change from every 3 weeks to every 4 weeks starting at cycle 5.
- ‡ intravenous infusion over 60 minutes [see Dosage and Administration (2.3)].
- § if patients receive fewer than 4 cycles of platinum-based chemotherapy, the remaining cycles of IMJUDO (up to a total of 5) should be given after the platinum-based chemotherapy phase, in combination with durvalumab, every 4 weeks.
- ¶ optional pemetrexed therapy from week 12 until disease progression or intolerable toxicity for patients with non-squamous disease who received treatment with pemetrexed and carboplatin/cisplatin.
Cycle:
1
2
3
4
5
6
7
8
X
X
X
X
X
X
X
X
X
X
X
X
X
Chemotherapy
X
X
X
X
X¶
X¶
X¶
X¶
Table 3: Recommended Regimen and Dosage - * Refer to the Prescribing Information for dosing information.
Tumor Histology
Patient Weight
IMJUDO
Dosage
Durvalumab*
Dosage
Platinum-based
Chemotherapy Regimen*
Non-Squamous
≥ 30 kg
75 mg
1,500 mg
- carboplatin & nab-paclitaxel
OR
- carboplatin or cisplatin & pemetrexed
< 30 kg
1 mg/kg
20 mg/kg
Squamous
≥ 30 kg
75 mg
1,500 mg
- carboplatin & nab-paclitaxel
OR
- carboplatin or cisplatin & gemcitabine
< 30 kg
1 mg/kg
20 mg/kg
2.2 Dosage Modifications for Adverse Reactions
No dose reduction for treatment is recommended. In general, withhold treatment regimen for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue treatment regimen for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids.
Recommended treatment modifications are presented in Table 4.
Table 4. Recommended Dosage Modifications for Adverse Reactions - * Based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
- † Resume in patients with complete or partial resolution (Grade 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating steroids or an inability to reduce corticosteroid dose to 10 mg of prednisone or less per day (or equivalent) within 12 weeks of initiating corticosteroids.
- ‡ If AST and ALT are less than or equal to ULN at baseline in patients with liver involvement, withhold or permanently discontinue durvalumab based on recommendations for hepatitis with no liver involvement.
Adverse Reaction
Severity*
Dosage Modification
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)]
Pneumonitis
Grade 2
Withhold†
Grade 3 or 4
Permanently discontinue
Colitis
Grade 2
Withhold†
Grade 3 or 4
Permanently discontinue
Intestinal perforation
Any grade
Permanently discontinue
Hepatitis with no tumor involvement of the liver
ALT or AST increases to more than 3 and up to 8 times the ULN
or
total bilirubin increases to more than 1.5 and up to 3 times ULN
Withhold†
ALT or AST increases to more than 8 times ULN
or
total bilirubin increases to more than 3 times the ULN
Permanently discontinue
Hepatitis with tumor involvement of the liver‡
AST or ALT is more than 1 and up to 3 times ULN at baseline and increases to more than 5 and up to 10 times ULN
or
AST or ALT is more than 3 and up to 5 times ULN at baseline and increases to more than 8 and up to 10 times ULN
Withhold†
AST or ALT increases to more than 10 times ULN
or
Total bilirubin increases to more than 3 times ULN
Permanently discontinue
Endocrinopathies
Grade 3 or 4
Withhold until clinically stable or permanently discontinue depending on severity
Nephritis with Renal Dysfunction
Grade 2 or 3 increased blood creatinine
Withhold†
Grade 4 increased blood creatinine
Permanently discontinue
Exfoliative Dermatologic Conditions
Suspected SJS, TEN, or DRESS
Withhold†
Confirmed SJS, TEN, or DRESS
Permanently discontinue
Myocarditis
Grade 2, 3, or 4
Permanently discontinue
Neurological Toxicities
Grade 2
Withhold†
Grade 3 or 4
Permanently discontinue
Other Adverse Reactions
Infusion-related reactions [see Warnings and Precautions (5.2)]
Grade 1 or 2
Interrupt or slow the rate of infusion
Grade 3 or 4
Permanently discontinue
ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson Syndrome, TEN = toxic epidermal necrolysis, ULN = upper limit normal
2.3 Preparation and Administration
Preparation
- Visually inspect drug product for particulate matter and discoloration. Discard if the solution is cloudy, discolored, or visible particles are observed.
- Do not shake the vial.
- Withdraw the required volume from the vial(s) of IMJUDO and discard the vial with any unused portion.
- Transfer into an intravenous bag containing 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP and dilute to a concentration between 0.1 mg/mL and 10 mg/mL. Mix diluted solution by gentle inversion. Do not shake the solution.
Storage of Diluted IMJUDO Infusion Solution
- IMJUDO does not contain a preservative. Administer infusion solution immediately once prepared. If infusion solution is not administered immediately and needs to be stored, the total time from preparation to the start of administration should not exceed:
24 hours in a refrigerator at 2°C to 8°C (36°F to 46°F)
24 hours at room temperature up to 30°C (86°F)
- Do not freeze.
- Do not shake.
Administration
- Administer IMJUDO infusion solution intravenously over 60 minutes through an intravenous line containing a sterile, low-protein binding 0.2 or 0.22 micron filter.
- Use separate infusion bags and filters for each drug product.
IMJUDO In Combination with Other Products
- Administer all drug products as separate intravenous infusions.
- Do not co-administer other drugs through the same infusion line.
- For platinum-based chemotherapy, refer to Prescribing Information for administration information.
- For pemetrexed treatment, refer to Prescribing Information for administration information.
Combination Regimens: Order of Infusions
IMJUDO in Combination with Durvalumab
- Infuse IMJUDO, followed by durvalumab on the same day of dosing.
IMJUDO in Combination with Durvalumab and Platinum-based Chemotherapy
- Infuse IMJUDO first, followed by durvalumab and then platinum-based chemotherapy on the day of dosing.
IMJUDO in Combination with Durvalumab and Pemetrexed Therapy
- Infuse IMJUDO first, followed by durvalumab and then pemetrexed treatment on the day of dosing.
Combination Regimens: Infusion Instructions
IMJUDO in Combination with Durvalumab
- Observe patient for 60 minutes following completion of IMJUDO infusion [see Warnings and Precautions (5.2)]. Then administer durvalumab as a separate intravenous infusion over 60 minutes
IMJUDO in Combination with Durvalumab and Platinum-based Chemotherapy/ Pemetrexed Therapy
Cycle 1:
Infuse IMJUDO over one hour. One to two hours after completion of IMJUDO infusion, infuse durvalumab over one hour. One to two hours after completion of durvalumab infusion, administer platinum-based chemotherapy.
Subsequent Cycles:
If there are no infusion reactions during cycle 1, subsequent cycles of durvalumab can be given immediately after IMJUDO. The time between the end of the durvalumab infusion and the start of chemotherapy can be reduced to 30 minutes.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
IMJUDO is a monoclonal antibody that blocks T-cell inhibitory signals induced by the CTLA-4 pathway, thereby removing inhibition of the immune response. In combination with durvalumab, a PD-L1 inhibitor, these drugs have the potential for induction of immune-mediated adverse reactions. Immune-mediated adverse reactions listed herein may not be inclusive of all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting IMJUDO in combination with durvalumab. While immune-mediated adverse reactions usually manifest during treatment, immune-mediated adverse reactions can also manifest after discontinuation of IMJUDO and/or durvalumab.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of IMJUDO in combination with durvalumab. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue IMJUDO and durvalumab depending on severity [see Dosage and Administration (2.2)]. In general, if combination of IMJUDO and durvalumab requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
IMJUDO in combination with durvalumab can cause immune-mediated pneumonitis, which may be fatal.
IMJUDO with Durvalumab
Immune-mediated pneumonitis occurred in 1.3% (5/388) of patients receiving IMJUDO in combination with durvalumab, including fatal (0.3%) and Grade 3 (0.2%) adverse reactions. Events resolved in 3 of the 5 patients and resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in all patients; of these, 4 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). One patient (1/5) required other immunosuppressants.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated pneumonitis occurred in 3.5% (21/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including fatal (0.5%) and Grade 3 (1%) adverse reactions. Events resolved in 11 of the 21 patients and resulted in permanent discontinuation in 7 patients. Systemic corticosteroids were required in all patients with immune-mediated pneumonitis, while 1 patient (1/21) required other immunosuppressants.
Immune-Mediated Colitis
IMJUDO in combination with durvalumab and platinum-based chemotherapy can cause immune-mediated colitis, which may be fatal.
IMJUDO in combination with durvalumab can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
IMJUDO with Durvalumab
Immune-mediated colitis or diarrhea occurred in 6% (23/388) of patients receiving IMJUDO in combination with durvalumab, including Grade 3 (3.6%) adverse reactions. Events resolved in 22 of the 23 patients and resulted in permanent discontinuation in 5 patients. All patients received systemic corticosteroids, and 20 of the 23 patients received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Three patients also received other immunosuppressants.
Intestinal perforation has been observed in other studies of IMJUDO in combination with durvalumab.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated colitis occurred in 6.5% (39/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including fatal (0.2%) and Grade 3 (2.5%) adverse reactions. Events resolved in 33 of the 39 patients and resulted in permanent discontinuation in 11 patients. Systemic corticosteroids were required in all patients with immune-mediated colitis, while 4 patients (4/39) required other immunosuppressants.
Intestinal perforation and large intestine perforation were reported in 0.1% of patients receiving IMJUDO in combination with durvalumab.
Immune-Mediated Hepatitis
IMJUDO in combination with durvalumab can cause immune-mediated hepatitis, which may be fatal.
IMJUDO with Durvalumab
Immune-mediated hepatitis occurred in 7.5% (29/388) of patients receiving IMJUDO in combination with durvalumab, including fatal (0.8%), Grade 4 (0.3%), and Grade 3 (4.1%) adverse reactions. Events resolved in 12 of the 29 patients and resulted in permanent discontinuation in 9 patients. Systemic corticosteroids were required in all 29 patients and all 29 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Eight patients (8/29) required other immunosuppressants.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated hepatitis occurred in 3.9% (23/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including fatal (0.3%), Grade 4 (0.5%), and Grade 3 (2%) adverse reactions. Events resolved in 12 of the 23 patients and resulted in permanent discontinuation in 27 patients. Systemic corticosteroids were required in all patients with immune-mediated hepatitis, while 2 patients (2/23) required use of other immunosuppressants.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMJUDO in combination with durvalumab can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold or permanently discontinue IMJUDO in combination with durvalumab based on the severity [see Dosage and Administration (2.2)].
IMJUDO with Durvalumab
Immune-mediated adrenal insufficiency occurred in 1.5% (6/388) of patients receiving IMJUDO in combination with durvalumab, including Grade 3 (0.3%) adverse reactions. Events resolved in 2 of the 6 patients. Systemic corticosteroids were required in all 6 patients, and of these, 1 patient required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day).
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated adrenal insufficiency occurred in 2.2% (13/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.8%) adverse reactions. Events resolved in 2 of the 13 patients and resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in all patients with adrenal insufficiency. One patient also required endocrine therapy.
Hypophysitis: IMJUDO in combination with durvalumab can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated. Withhold or permanently discontinue IMJUDO in combination with durvalumab depending on severity [see Dosage and Administration (2.2)].
IMJUDO with Durvalumab
Immune-mediated hypophysitis/hypopituitarism occurred in 1% (4/388) of patients receiving IMJUDO in combination with durvalumab. Events resolved in 2 of the 4 patients. Systemic corticosteroids were required in 3 patients, and of these, 1 patient received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Two patients also required endocrine therapy.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated hypophysitis occurred in 1.3% (8/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.5%) adverse reactions. Events resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in 6 patients with immune-mediated hypophysitis; of these, 2 of the 8 patients received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Four patients also required endocrine therapy.
Thyroid Disorders: IMJUDO in combination with durvalumab can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated. Withhold or discontinue IMJUDO in combination with durvalumab based on the severity [see Dosage and Administration (2.2)].
Thyroiditis:
IMJUDO with Durvalumab
Immune-mediated thyroiditis occurred in 1.5% (6/388) of patients receiving IMJUDO in combination with durvalumab. Events resolved in 2 of the 6 patients. Systemic corticosteroids were required in 2 patients (2/6) with immune-mediated thyroiditis; of these, 1 patient required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). All patients required other therapy including hormone replacement therapy, thiamazole, carbimazole, propylthiouracil, perchlorate, calcium channel blocker, or beta-blocker.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated thyroiditis occurred in 1.2% (7/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy. Events resolved in 2 of the 7 patients and one resulted in permanent discontinuation. Systemic corticosteroids were required in 2 patients (2/7) with immune-mediated thyroiditis, while all patients required endocrine therapy.
Hyperthyroidism:
IMJUDO with Durvalumab
Immune-mediated hyperthyroidism occurred in 4.6% (18/388) of patients receiving IMJUDO in combination with durvalumab, including Grade 3 (0.3%) adverse reactions. Events resolved in 15 of the 18 patients. Two patients (2/18) required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Seventeen patients required other therapy (thiamazole, carbimazole, propylthiouracil, perchlorate, calcium channel blocker, or beta-blocker).
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated hyperthyroidism occurred in 5% (30/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.2%) adverse reactions. Events resolved in 21 of the 30 patients. Systemic corticosteroids were required in 5 patients (5/30) with immune-mediated hyperthyroidism, while 28 patients (28/30) required endocrine therapy.
Hypothyroidism:
IMJUDO with Durvalumab
Immune-mediated hypothyroidism occurred in 11% (42/388) of patients receiving IMJUDO in combination with durvalumab. Events resolved in 5 of the 42 patients. One patient received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). All patients required other therapy (thiamazole, carbimazole, propylthiouracil, perchlorate, calcium channel blocker, or beta-blocker).
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated hypothyroidism occurred in 8.6% (51/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.5%) adverse reactions. Systemic corticosteroids were required in 2 patients (2/51) and all patients required endocrine therapy.
Type 1 Diabetes Mellitus, Which Can Present with Diabetic Ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue IMJUDO in combination with durvalumab based on the severity [see Dosage and Administration (2.2)].
IMJUDO with Durvalumab
Two patients (0.5%, 2/388) had events of hyperglycemia requiring insulin therapy that had not resolved at last follow-up.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated Type 1 Diabetes Mellitus occurred in 0.5% (3/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.3%) adverse reactions. All patients required endocrine therapy.
Immune-Mediated Nephritis with Renal Dysfunction
IMJUDO in combination with durvalumab can cause immune-mediated nephritis.
IMJUDO with Durvalumab
Immune-mediated nephritis occurred in 1% (4/388) of patients receiving IMJUDO in combination with durvalumab, including Grade 3 (0.5%) adverse reactions. Events resolved in 3 of the 4 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated nephritis; of these, 3 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day).
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated nephritis occurred in 0.7% (4/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.2%) adverse reactions. Events resolved in 1 of the 4 patients and resulted in permanent discontinuation in 3 patients. Systemic corticosteroids were required in all patients with immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMJUDO in combination with durvalumab can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with CTLA-4 and PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue IMJUDO in combination with durvalumab depending on severity [see Dosage and Administration (2.2)].
IMJUDO with Durvalumab
Immune-mediated rash or dermatitis occurred in 4.9% (19/388) of patients receiving IMJUDO in combination with durvalumab, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions. Events resolved in 13 of the 19 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated rash or dermatitis; of these, 12 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). One patient received other immunosuppressants.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Immune-mediated rash or dermatitis occurred in 7.2% (43/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.3%) adverse reactions. Events resolved in 32 of the 43 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated rash or dermatitis.
Immune-Mediated Pancreatitis
IMJUDO in combination with durvalumab can cause immune-mediated pancreatitis.
IMJUDO with Durvalumab
Immune-mediated pancreatitis occurred in 2.3% (9/388) of patients receiving IMJUDO in combination with durvalumab, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions. Events resolved in 6 of the 9 patients. Systemic corticosteroids were required in all 9 patients and of these, 7 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day).
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMJUDO in combination with durvalumab or were reported with the use of other immune-checkpoint inhibitors.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatica.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, and immune thrombocytopenia.
5.2 Infusion-Related Reactions
IMJUDO in combination with durvalumab can cause severe or life-threatening infusion-related reactions.
Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMJUDO and durvalumab based on the severity [see Dosage and Administration (2.2)]. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
IMJUDO with Durvalumab
Infusion-related reactions occurred in 10 (2.6%) patients receiving IMJUDO in combination with durvalumab.
IMJUDO with Durvalumab and Platinum-Based Chemotherapy
Infusion-related reactions occurred in 2.9% (17/596) of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy, including Grade 3 (0.3%) adverse reactions.
5.3 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, IMJUDO can cause fetal harm when administered to a pregnant woman. In animal studies, CTLA-4 blockade is associated with higher incidence of pregnancy loss.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with IMJUDO and for 3 months after the last dose of IMJUDO [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling.
- Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)].
- Infusion-Related Reactions [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the Warnings and Precautions reflect exposure to IMJUDO 300 mg in combination with durvalumab 1,500 mg in 388 patients in HIMALAYA. In the HIMALAYA study patients received IMJUDO 300 mg administered as a single intravenous infusion in combination with durvalumab 1,500 mg on the same day, followed by durvalumab every 4 weeks.
The data also reflects exposure to IMJUDO 75 mg in combination with durvalumab 1,500 mg and histology-based platinum chemotherapy regimens in the pooled safety population (N=596) of 330 patients in POSEIDON [see Clinical Studies (14.1)], and 266 patients in CASPIAN who received up to four cycles of platinum-etoposide plus durvalumab 1,500 mg with tremelimumab-actl 75 mg every 3 weeks, followed by durvalumab 1,500 mg every 4 weeks (an unapproved regimen for extensive-stage small cell lung cancer). Of these patients, 64% received the maximum of 5 doses of IMJUDO and 79% received at least 4 doses.
In this pooled safety population, the most common (> 20%) adverse reactions were nausea (37%), decreased appetite (25%), and fatigue (22%). In this pooled safety population, the most common Grade 3 or 4 (> 10%) laboratory abnormalities were neutropenia (39%), leukopenia (21%), lymphocytopenia (20%), anemia (20%), hyponatremia (14%), lipase increased (12%), and thrombocytopenia (11%).
The data described in this section reflect exposure to IMJUDO in patients with uHCC included in the HIMALAYA study and in patients with metastatic NSCLC enrolled in the POSEIDON study.
Hepatocellular Carcinoma
Unresectable HCC - HIMALAYA
The safety of IMJUDO administered in combination with durvalumab was evaluated in a total of 388 patients with uHCC in HIMALAYA, a randomized, open-label, multicenter study [see Clinical Studies (14.1)]. Patients received IMJUDO 300 mg administered as a single intravenous infusion in combination with durvalumab 1,500 mg on the same day, followed by durvalumab every 4 weeks or sorafenib 400 mg given orally twice daily.
Serious adverse reactions occurred in 41% of patients who received IMJUDO in combination with durvalumab. Serious adverse reactions in > 1% of patients included hemorrhage (6%), diarrhea (4%), sepsis (2.1%), pneumonia (2.1%), rash (1.5%), vomiting (1.3%), acute kidney injury (1.3%), and anemia (1.3%). Fatal adverse reactions occurred in 8% of patients who received IMJUDO in combination with durvalumab, including death (1%), hemorrhage intracranial (0.5%), cardiac arrest (0.5%), pneumonitis (0.5%), hepatic failure (0.5%), and immune-mediated hepatitis (0.5%). The most common adverse reactions (occurring in ≥ 20% of patients) were rash, diarrhea, fatigue, pruritus, musculoskeletal pain, and abdominal pain.
Permanent discontinuation of the treatment regimen due to an adverse reaction occurred in 14% of patients; the most common adverse reactions leading to treatment discontinuation (≥ 1%) were hemorrhage (1.8%), diarrhea (1.5%), AST increased (1%), and hepatitis (1%).
Dosage interruptions or delay of the treatment regimen due to an adverse reaction occurred in 35% of patients. Adverse reactions which required dosage interruption or delay in ≥ 1% of patients included ALT increased (3.6%), diarrhea (3.6%), rash (3.6%), amylase increased (3.4%), AST increased (3.1%), lipase increased (2.8%), pneumonia (1.5%), hepatitis (1.5%), pyrexia (1.5%), anemia (1.3%), thrombocytopenia (1%), hyperthyroidism (1%), pneumonitis (1%), and blood creatinine increased (1%).
Table 5 summarizes the adverse reactions that occurred in patients treated with IMJUDO in combination with durvalumab in the HIMALAYA study.
Table 5. Adverse Reactions Occurring in ≥ 10% Patients in the HIMALAYA study IMJUDO and Durvalumab
(N=388)Sorafenib
(N=374)Adverse Reaction All Grades (%) Grade 3-4 (%) All Grades (%) Grade 3-4 (%) - * Represents a composite of multiple related terms.
Gastrointestinal disorders
Diarrhea*
27
6
45
4.3
Abdominal pain*
20
1.8
24
4
Nausea
12
0
14
0
Skin and subcutaneous tissue disorders
Rash*
32
2.8
57
12
Pruritus
23
0
6
0.3
Metabolism and nutrition disorders
Decreased appetite
17
1.3
18
0.8
General disorders and administration site conditions
Fatigue*
26
3.9
30
6
Pyrexia*
13
0.3
9
0.3
Psychiatric disorders
Insomnia
10
0.3
4.3
0
Endocrine disorders
Hypothyroidism*
14
0
6
0
Musculoskeletal and Connective Tissue Disorders
Musculoskeletal pain*
22
2.6
17
0.8
Table 6 summarizes the laboratory abnormalities that occurred in patients treated with IMJUDO in combination with durvalumab in the HIMALAYA study.
Table 6. Laboratory Abnormalities Worsening from Baseline Occurring in ≥ 20% of Patients in the HIMALAYA study IMJUDO and Durvalumab
Sorafenib
Laboratory Abnormality
Any grade
(%)
Grade 3 or 4
(%)
Any grade
(%)
Grade 3 or 4
(%)
Chemistry
Aspartate Aminotransferase increased
63
27
55
21
Alanine Aminotransferase increased
56
18
53
12
Sodium decreased
46
15
40
11
Bilirubin increased
41
8
47
11
Alkaline Phosphatase increased
41
8
44
5
Glucose increased
39
14
29
4
Calcium decreased
34
0
43
0.3
Albumin decreased
31
0.5
37
1.7
Potassium increased
28
3.8
21
2.6
Creatinine increased
21
1.3
15
0.9
Hematology
Hemoglobin decreased
52
4.8
40
6
Lymphocytes decreased
41
11
39
10
Platelets decreased
29
1.6
35
3.1
Leukocytes decreased
20
0.8
30
1.1
Non-Small Cell Lung Cancer
Metastatic NSCLC – POSEIDON
The safety of IMJUDO in combination with durvalumab and platinum-based chemotherapy in patients with metastatic NSCLC was evaluated in POSEIDON (NCT03164616), a randomized, open-label, multicenter, active-controlled trial. A total of 330 patients received IMJUDO (≥ 30 kg body weight received 75 mg and ≤ 30kg body weight received 1 mg/kg) in combination with durvalumab 1,500 mg and histology-based platinum chemotherapy regimens [see Clinical Studies (14.2)]. Of these patients, 66% received up to the maximum 5 doses of IMJUDO and 79% received at least 4 doses. Treatment was continued with durvalumab as a single agent (or with durvalumab and histology-based pemetrexed for non-squamous patients, based on the investigator’s decision) until disease progression or unacceptable toxicity. The trial excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants [see Clinical Studies (14.2)].
The median age of patients who received IMJUDO in combination with durvalumab and platinum-based chemotherapy was 63 years (range: 27 to 87); 80% male; 61% White, 29% Asian, 58% former smoker, 25% current smoker, and 68% ECOG performance of 1.
Serious adverse reactions occurred in 44% of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonia (11%), anemia (5%), diarrhea (2.4%), thrombocytopenia (2.4%), pyrexia (2.4%), and febrile neutropenia (2.1%). Fatal adverse reactions occurred in a total of 4.2% of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy. These include hepatitis, nephritis, myocarditis, pancreatitis (all in the same patient), death (2 patients), sepsis (2 patients), pneumonitis (2 patients), acute kidney injury (2 patients), febrile neutropenia (1 patient), chronic obstructive pulmonary disease (1 patient), dyspnea (1 patient), sudden death (1 patient), and ischemic stroke (1 patient).
Permanent discontinuation of IMJUDO or durvalumab due to an adverse reaction occurred in 17% of the patients. Adverse reactions which resulted in permanent discontinuation of IMJUDO or durvalumab in > 2% of patients included pneumonia.
Dosage interruptions or delay of IMJUDO and durvalumab due to an adverse reaction occurred in 41% of patients. Adverse reactions which required dosage interruption or delay of IMJUDO and durvalumab in > 1% of patients included anemia, leukopenia/white blood cell count decreased, pneumonia, pneumonitis, colitis, diarrhea, hepatitis, rash, asthenia, amylase increased, alanine aminotransferase increased, aspartate aminotransferase increased, lipase increased, neutropenia/neutrophil count decreased, and thrombocytopenia/platelet count decreased.
The most common adverse reactions (occurring in ≥ 20% of patients) were nausea, fatigue, musculoskeletal pain, decreased appetite, rash, and diarrhea. Grade 3 or 4 laboratory abnormalities (≥ 10%) were neutropenia, anemia, leukopenia, lymphocytopenia, lipase increased, hyponatremia and thrombocytopenia.
Table 7 summarizes the adverse reactions in POSEIDON.
Table 7. Adverse Reactions (≥ 10%) in Patients with NSCLC Who Received IMJUDO in the POSEIDON Study - * Includes cough and productive cough.
- † Includes mucosal inflammation and stomatitis.
- ‡ Includes blood thyroid stimulating hormone increased and hypothyroidism.
- § Includes eczema, erythema, dermatitis, drug eruption, erythema multiforme, pemphigoid, rash, rash maculo-papular, rash papular, rash pruritic and rash pustular.
- ¶ Includes asthenia and fatigue.
- # Includes body temperature increased, hyperpyrexia, hyperthermia, and pyrexia.
- Þ Includes face edema, localized edema, and edema peripheral.
- ß Includes arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain, spinal pain.
- à Includes lower respiratory tract infection, pneumocystis jirovecii pneumonia, pneumonia, pneumonia aspiration, pneumonia bacterial.
- è Includes laryngitis, nasopharyngitis, pharyngitis, rhinitis, sinusitis, tonsillitis, tracheobronchitis and upper respiratory tract infection.
- ð Includes headache, migraine.
IMJUDO with durvalumab and platinum-based chemotherapy
N = 330
Platinum-based chemotherapy
N = 333
Adverse Reaction
All Grades (%)
Grade 3 or 4 (%)
All Grades (%)
Grade 3 or 4 (%)
Respiratory, thoracic and mediastinal disorders
Cough/Productive Cough*
12
0
8
0.3
Gastrointestinal disorders
Nausea
42
1.8
37
2.1
Diarrhea
22
1.5
15
1.5
Constipation
19
0
24
0.6
Vomiting
18
1.2
14
1.5
Stomatitis†
10
0
6
0.3
Endocrine disorders
Hypothyroidism‡
13
0
2.1
0
Skin and subcutaneous tissue disorders
Rash§
27
2.4
10
0.6
Alopecia
10
0
6
0
Pruritus
11
0
4.5
0
General disorders and administration site conditions
Fatigue/Asthenia¶
36
5
32
4.5
Pyrexia#
19
0
8
0
EdemaÞ
10
0
10
0.6
Musculoskeletal and connective tissue disorders
Musculoskeletal Painß
29
0.6
22
1.5
Metabolism and nutrition disorders
Decreased appetite
28
1.5
25
1.2
Infections and Infestations
Pneumoniaà
17
8
12
4.2
Upper respiratory tract infectionsè
15
0.6
9
0.9
Nervous system disorders
Headacheð
11
0
8
0.6
Table 8 summarizes the laboratory abnormalities in POSEIDON.
Table 8: Select Laboratory Abnormalities (≥ 10%) That Worsened from Baseline in Patients with NSCLC Who Received IMJUDO in the POSEIDON Study Laboratory Abnormality
IMJUDO with Durvalumab and Platinum-based chemotherapy
Platinum-based chemotherapy
- All Grades
- (%)
- Grade 3 or 4
- (%)
All Grades
(%)
Grade 3 or 4
(%)
Chemistry
Lipase increased
35
14
25
5
Hyponatremia
55
13
50
11
Hypernatremia
15
0
14
0
Amylase increased
41
9
25
6
Hypokalemia
21
7
17
2.8
Hyperglycemia
42
6
37
3.1
Increased ALT
64
6
56
4.7
Increased AST
63
5
55
2.2
Blood creatinine increased
89
4.0
83
1.9
Increased Alkaline Phosphatase
33
3.4
26
1.2
Gamma Glutamyl Transferase increased
38
2.2
35
4.7
Hyperkalemia
49
2.2
35
2.8
Albumin decreased
27
1.9
18
0.9
Hypocalcemia
58
0.9
49
0.9
Hypomagnesemia
12
4
23
0
Bilirubinemia
16
0.9
8
0.3
Hematology
Neutropenia
71
37
69
32
Anemia
84
24
84
25
Leukopenia
77
21
81
18
Lymphocytopenia
67
20
60
19
Thrombocytopenia
53
11
54
12
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk summary
Based on findings from animal studies and its mechanism of action, IMJUDO can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of IMJUDO in pregnant women. In animal studies, CTLA-4 blockade is associated with increased risk of immune-mediated rejection of the developing fetus and fetal death (see Data).
Human immunoglobulin G2 (IgG2) is known to cross the placental barrier; therefore, IMJUDO has the potential to be transmitted from the mother to the developing fetus. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In a reproduction study, administration of tremelimumab-actl to pregnant cynomolgus monkeys during the period of organogenesis was not associated with maternal toxicity or effects on embryo-fetal development at exposure levels approximately 4 to 31-times higher than those observed at a recommended dose range of 75 mg to 300 mg based on area under the curve (AUC). CTLA-4 plays a role in maintaining maternal immune tolerance to the fetus to preserve pregnancy and in immune regulation of the newborn. In a murine model of pregnancy, CTLA-4 blockade resulted in increased resorptions and reduced live fetuses. Mated genetically engineered mice heterozygous for CTLA-4 (CTLA-4+/-) gave birth to CTLA-4+/- offspring and offspring deficient in CTLA-4 (homozygous negative, CTLA-4-/-) that appeared healthy at birth. The CTLA-4-/- homozygous negative offspring developed signs of a lymphoproliferative disorder and died by 3 to 4 weeks of age with multiorgan tissue destruction. Based on its mechanism of action, fetal exposure to tremelimumab-actl may increase the risk of developing immune-mediated disorders or altering the normal immune response.
8.2 Lactation
Risk Summary
There are no data on the presence of tremelimumab-actl in human milk, its effects on a breastfed child, or on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to IMJUDO are unknown. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with IMJUDO and for 3 months after the last dose. Refer to the Prescribing Information for agents administered in combination with IMJUDO for breastfeeding recommendations, as appropriate.
8.3 Females and Males of Reproductive Potential
IMJUDO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating treatment with IMJUDO.
Contraception
Advise females of reproductive potential to use effective contraception during treatment with IMJUDO and for 3 months after the last dose. Refer to the Prescribing Information for the agents administered in combination with IMJUDO for recommended contraception duration, as appropriate.
8.4 Pediatric Use
The safety and effectiveness of IMJUDO have not been established in pediatric patients. Safety and efficacy were assessed but not established in a multi-center, open-label study (NCT03837899) in which 41 pediatric patients aged 1 to < 17 years with advanced solid tumors received IMJUDO in combination with durvalumab. No new safety signals were observed in pediatric patients in this study.
Tremelimumab-actl systemic exposure in pediatric patients ≥ 35 kg was within the range of the values previously observed in adults given the same weight-based dose, whereas the systemic exposure in pediatric patients < 35 kg was lower than that of adults.
8.5 Geriatric Use
Of the 393 patients with uHCC treated with IMJUDO in combination with durvalumab, 50% of patients were 65 years or older and 13% of patients were 75 years or older. No overall differences in safety or efficacy of IMJUDO have been observed between patients 65 years or older and younger adult patients.
Of the 330 patients with metastatic NSCLC treated with IMJUDO in combination with durvalumab and platinum-based chemotherapy, 143 (43%) patients were 65 years or older and 35 (11%) patients were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
-
11 DESCRIPTION
Tremelimumab-actl, a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blocking human IgG2 monoclonal antibody, is produced by recombinant DNA technology in NS0 cell suspension culture and has a molecular weight of 149 kDa.
IMJUDO (tremelimumab-actl) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution, in a single-dose vial for intravenous infusion after dilution. IMJUDO contains tremelimumab-actl at a concentration of 20 mg/mL in either a 25 mg/1.25 mL or a 300 mg/15 mL single-dose vial.
Each mL contains 20 mg of tremelimumab-actl, and edetate disodium (0.09 mg), histidine (0.68 mg), L‑histidine hydrochloride monohydrate (3.3 mg), polysorbate 80 (0.2 mg), trehalose (76 mg), and Water for Injection, USP. The pH is approximately 5.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CTLA-4 is a negative regulator of T-cell activity. Tremelimumab-actl is a monoclonal antibody that binds to CTLA-4 and blocks the interaction with its ligands CD80 and CD86, releasing CTLA-4-mediated inhibition of T-cell activation. In synergistic mouse tumor models, blocking CTLA-4 activity resulted in decreased tumor growth and increased proliferation of T cells in tumors.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of tremelimumab-actl have not been fully characterized.
12.3 Pharmacokinetics
The pharmacokinetics of tremelimumab-actl was studied in patients with other solid tumors following administration of doses 1 mg/kg, 3 mg/kg, and 10 mg/kg (1- to 10-times the approved recommended dosage) administered once every 4 weeks for 4 doses. The pharmacokinetics of tremelimumab-actl as a single dose of 300 mg were evaluated in patients with HCC.
The AUC of tremelimumab-actl increased proportionally from 1 mg/kg to 10 mg/kg every 4 weeks (1 to 10-times the approved recommended dosage) and steady state was achieved at approximately 12 weeks.
Distribution
The geometric mean (% coefficient of variation [CV%]) of tremelimumab-actl for central (V1) and peripheral (V2) volume of distribution was 3.45 (24%) and 2.66 (34%) L, respectively.
Elimination
The geometric mean (CV%) terminal half-life of tremelimumab-actl was 16.9 days (19%) after a single dose and 18.2 days (19%) during steady state. The geometric mean (CV%) clearance of tremelimumab-actl was 0.286 L/day (32%) after a single dose and 0.263 L/day (32%) during steady state.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of tremelimumab-actl based on body weight (34 to149 kg), age (18 to 87 years), sex, race (White, Black, Asian, Native Hawaiian, Pacific Islander, or American Indian), serum albumin levels (0.3 to 396 g/L), lactate dehydrogenase levels (12 to 5570 U/L), soluble PD-L1 (67 to 349 pg/mL), tumor type (NSCLC, HCC), organ dysfunction including mild to moderate renal impairment (CLcr 30 to 89 mL/min), and mild to moderate hepatic impairment (bilirubin < 3 x ULN and any AST).
The effect of severe renal impairment (CLcr 15 to 29 mL/min) or severe hepatic impairment (bilirubin > 3 x ULN and any AST) on the pharmacokinetics of tremelimumab-actl is unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of tremelimumab-actl.
In the HIMALAYA study, of the 182 patients who were treated with a single dose of tremelimumab-actl in combination with durvalumab once in every 4 weeks therapy and evaluable for the presence of ADAs against tremelimumab-actl at predose week 0 and week 4, 11% (20/182) of patients tested positive for anti-tremelimumab-actl antibodies. Among the 20 patients who tested positive for ADAs 40% (8/20) tested positive for neutralizing antibodies against tremelimumab-actl. There was no identified clinically significant effect of anti-tremelimumab-actl antibodies on the pharmacokinetics or safety of tremelimumab-actl; however, the effect of ADAs and neutralizing antibodies on the effectiveness of tremelimumab-actl is unknown.
In the POSEIDON study, of the 278 ADA-evaluable patients who were treated with IMJUDO 75 mg for up to five doses in combination with durvalumab 1,500 mg and platinum-based chemotherapy every 3 weeks and evaluated for presence of ADAs against tremelimumab-actl at pre-dose week 0, week 3, and week 12, 14% (38/278) of patients tested positive for anti-tremelimumab-actl antibodies. Among the 38 patients who tested positive for ADAs, 82% (31/38) tested positive for neutralizing antibodies against tremelimumab-actl. There was no identified clinically significant effect of anti-tremelimumab-actl antibodies on pharmacokinetics or safety of tremelimumab-actl, however, the effect of ADAs on effectiveness of tremelimumab-actl is unknown.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Hepatocellular Carcinoma (HCC)
Unresectable HCC - HIMALAYA
The efficacy of IMJUDO in combination with durvalumab was evaluated in the HIMALAYA study (NCT03298451), a randomized (1:1:1), open-label, multicenter study in patients with confirmed uHCC who had not received prior systemic treatment for HCC. Patients were randomized to one of two investigational arms (IMJUDO plus durvalumab or durvalumab) or sorafenib. Study treatment consisted of IMJUDO as a one-time single intravenous infusion of 300 mg in combination with durvalumab 1,500 mg on the same day, followed by durvalumab every 4 weeks; durvalumab 1,500 mg every 4 weeks; or sorafenib 400 mg given orally twice daily, until disease progression or unacceptable toxicity. The efficacy assessment of IMJUDO is based on patients randomized to the IMJUDO plus durvalumab arm versus the sorafenib arm. Randomization was stratified by macrovascular invasion (MVI) (yes or no), etiology of liver disease (hepatitis B virus vs. hepatitis C virus vs. others) and ECOG performance status (0 vs. 1).
The study enrolled patients with BCLC Stage C or B (not eligible for locoregional therapy). The study excluded patients with co-infection of viral hepatitis B and hepatitis C; active or prior documented gastrointestinal (GI) bleeding within 12 months; ascites requiring non-pharmacologic intervention within 6 months; hepatic encephalopathy within 12 months before the start of treatment; active or prior documented autoimmune or inflammatory disorders. Esophagogastroduodenoscopy was not mandated prior to enrollment but adequate endoscopic therapy, according to institutional standards, was required for patients with a history of esophageal variceal bleeding or those assessed as high risk for esophageal variceal bleeding by the treating physician.
Study treatment was permitted beyond disease progression if the patient was clinically stable and was deriving clinical benefit as determined by the investigator.
The major efficacy outcome measure was overall survival (OS) between the IMJUDO plus durvalumab arm versus the sorafenib arm. Additional efficacy outcomes were investigator-assessed progression-free survival (PFS), objective response rate (ORR) and duration of response (DoR) according to RECIST v1.1. Tumor assessments were conducted every 8 weeks for the first 12 months and then every 12 weeks thereafter.
The baseline demographics of the IMJUDO plus durvalumab and sorafenib arms were as follows: male (85%), age < 65 years (50%), median age of 65 years (range: 18 to 88 years), White (46%), Asian (49%), Black or African American (2%), Native Hawaiian or other Pacific Islander (0.1%), race Unknown (2%), Hispanic or Latino (5%), Not Hispanic or Latino (94%), ethnicity Unknown (1%), ECOG PS 0 (62%); Child-Pugh Class score A (99%), macrovascular invasion (26%), extrahepatic spread (53%), viral etiology hepatitis B (31%), hepatitis C (27%), uninfected (42%).
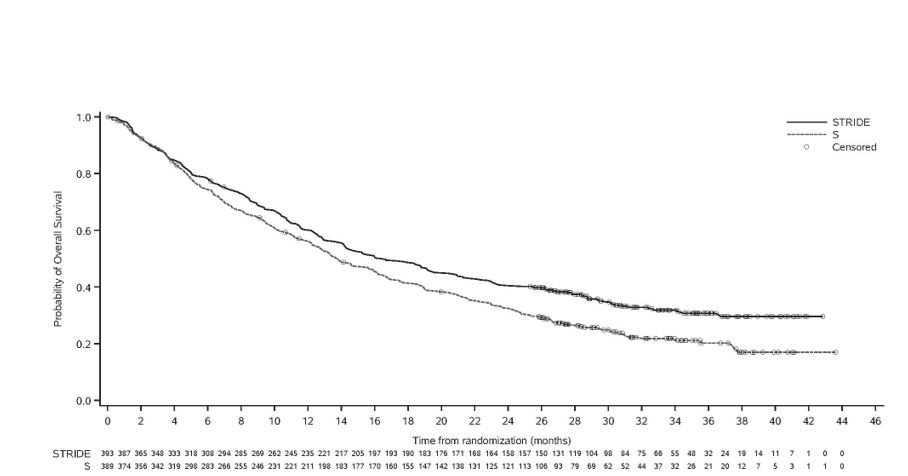
Efficacy results are presented in Table 9 and Figure 1.
Table 9. Efficacy Results for HIMALAYA Study - * HR (IMJUDO and durvalumab vs. sorafenib) based on the stratified Cox proportional hazard model.
- † Based on a stratified log-rank test.
- ‡ Based on a Lan-DeMets alpha spending function with O'Brien Fleming type boundary and the actual number of events observed, the boundary for declaring statistical significance for IMJUDO and durvalumab vs. sorafenib was 0.0398 (Lan and DeMets 1983).
- § Confirmed complete response or partial response.
- ¶ Based on Clopper-Pearson method.
Endpoint
IMJUDO and Durvalumab
(N=393)
Sorafenib
(N=389)
OS
Number of deaths (%)
262 (66.7)
293 (75.3)
- Median OS (months)
- (95% CI)
16.4
(14.2, 19.6)
13.8
(12.3, 16.1)
HR (95% CI) *
0.78 (0.66, 0.92)
0.0035
PFS
Number of events (%)
335 (85.2)
327 (84.1)
Median PFS (months)
(95% CI)
3.8
(3.7, 5.3)
4.1
(3.7, 5.5)
HR (95% CI) *
0.90 (0.77, 1.05)
ORR
20.1 (16.3, 24.4)
5.1 (3.2, 7.8)
- Complete Response n (%)
12 (3.1)
0
- Partial Response n (%)
67 (17.0)
20 (5.1)
DoR
- Median DoR (months)
- (95% CI)
22.3 (13.7, NR)
18.4 (6.5, 26.0)
- % with duration ≥ 6 months
82.3
78.9
- % with duration ≥ 12 months
65.8
63.2
CI=Confidence Interval, HR=Hazard Ratio, NR=Not Reached
Figure 1. Kaplan-Meier curve of OS
14.2 Metastatic NSCLC
Metastatic NSCLC – POSEIDON
The efficacy of IMJUDO in combination with durvalumab and platinum-based chemotherapy in previously untreated metastatic NSCLC patients with no sensitizing epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) genomic tumor aberrations was investigated in POSEIDON, a randomized, multicenter, active-controlled, open-label trial (NCT03164616). Eligible patients had Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 and must have had no prior chemotherapy or any other systemic therapy for metastatic NSCLC. Choice of platinum-based chemotherapy was at the Investigator’s discretion, taking into consideration the calculated creatinine clearance. Patients with active and/or untreated brain metastases; a history of active primary immunodeficiency; autoimmune disorders including active or prior documented autoimmune or inflammatory disorders; use of systemic immunosuppressants within 14 days before the first dose of the treatment except physiological dose of systemic corticosteroids were ineligible.
Randomization was stratified by tumor cells (TC) PD-L1 expression (TC ≥ 50% vs. TC < 50%), disease stage (Stage IVA vs. Stage IVB), and histology (non-squamous vs. squamous).
Patients were randomized 1:1:1 to receive IMJUDO in combination with durvalumab and platinum-based chemotherapy according to the regimens listed below, durvalumab and platinum-based chemotherapy (an unapproved regimen for metastatic NSCLC), or platinum-based chemotherapy. The evaluation of efficacy for metastatic NSCLC relied on comparison between:
- IMJUDO 75 mg (or 1mg/kg for patients < 30kg) with durvalumab 1,500 mg and platinum-based chemotherapy every 3 weeks for 4 cycles, followed by durvalumab 1,500 mg every 4 weeks as a single agent. A fifth dose of IMJUDO 75 mg (or 1mg/kg for patients < 30kg) was given at Week 16 in combination with durvalumab dose 6.
- Platinum-based chemotherapy every 3 weeks as monotherapy for 4 cycles. Patients could receive an additional 2 cycles (a total of 6 cycles post-randomization), as clinically indicated, at Investigator’s discretion.
Patients received IMJUDO and durvalumab in combination with one of the following platinum-based chemotherapy regimens:
- Non-squamous NSCLC
Pemetrexed 500 mg/m2 with carboplatin AUC 5-6 or cisplatin 75 mg/m2 every 3 weeks for 4 cycles
- Squamous NSCLC
Gemcitabine 1,000 or 1,250 mg/m2 on Days 1 and 8 with cisplatin 75 mg/m2 or carboplatin AUC 5-6 on Day 1 every 3 weeks for 4 cycles
- Non-squamous and Squamous NSCLC
Nab-paclitaxel 100 mg/m2 on Days 1, 8, and 15 with carboplatin AUC 5-6 on Day 1 every 3 weeks for 4 cycles
IMJUDO was given up to a maximum of 5 doses. Durvalumab and histology-based pemetrexed continued every 4 weeks until disease progression or unacceptable toxicity. Administration of durvalumab monotherapy was permitted beyond disease progression if the patient was clinically stable and deriving clinical benefit as determined by the Investigator. Patients with disease progression during durvalumab monotherapy were given the option to be retreated with 4 additional cycles of IMJUDO in combination with durvalumab. Tumor assessments were performed at Week 6, Week 12, and then every 8 weeks thereafter.
The major efficacy outcome measures were progression free survival (PFS) and overall survival (OS) of IMJUDO and durvalumab in combination with platinum-based chemotherapy compared to platinum-based chemotherapy alone. Additional efficacy outcome measures were overall response rate (ORR) and duration of response (DoR). PFS, ORR, and DoR were assessed using Blinded Independent Central Review (BICR) according to RECIST v1.1.
A total of 675 patients were randomized to receive either IMJUDO with durvalumab and platinum-based chemotherapy (n=338) or platinum-based chemotherapy (n=337). The median age was 63 years (range: 27 to 87), 46% of patients age ≥ 65 years, 77% male, 57% White, 34% Asian, 0.3% Native Hawaiian or Other Pacific Islander, 3% American Indian or Alaska Native, 2% Black or African American, 4% Other Race, 79% former or current smoker, 34% ECOG PS 0, and 66% ECOG PS 1. Thirty-six percent had squamous histology, 63% non-squamous histology, 29% PD-L1 expression TC ≥ 50%, 71% PD-L1 expression TC < 50%.
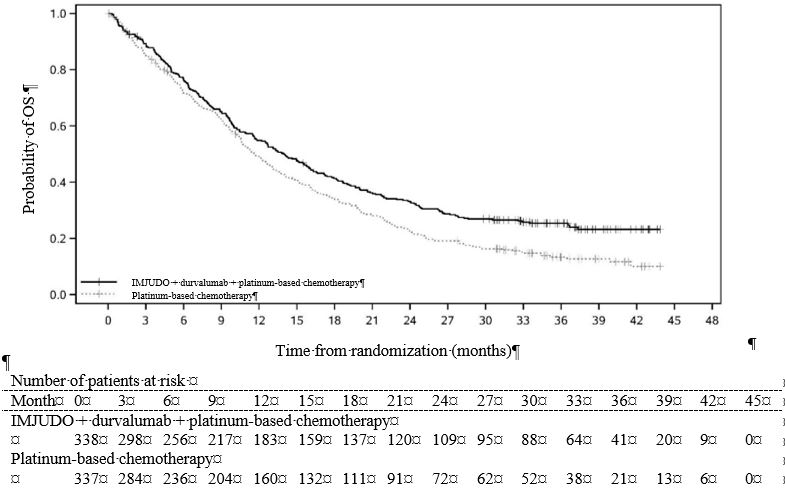
Efficacy results are summarized in Table 10 and Figure 2.
Table 10. Efficacy Results for POSEIDON - * PFS/OS results are based on planned analyses which occurred 25/45 months respectively after study initiation.
- † 2-sided p-values based on log-rank tests stratified by PD-L1, histology and disease stage and compared to a boundary value of 0.00735 for PFS and 0.00797 for OS.
- ‡ Confirmed responses with 95% Clopper-Pearson confidence intervals.
IMJUDO with durvalumab and platinum-based chemotherapy (n=338)
Platinum-based chemotherapy
(n=337)
OS*
- Number of deaths (%)
251 (74)
285 (85)
- Median OS (months)
- (95% CI)
14.0
(11.7, 16.1)
11.7
(10.5, 13.1)
HR (95% CI)
0.77 (0.65, 0.92)
- p-value†
0.00304
PFS*
- Number of events (%)
238 (70)
258 (77)
- Median PFS (months)
- (95% CI)
6.2
(5.0, 6.5)
4.8
(4.6, 5.8)
HR (95% CI)
0.72 (0.60, 0.86)
- p-value†
0.00031
ORR % (95% CI)‡
39 (34, 44)
24 (20, 29)
Median DoR (months)
- (95% CI)
9.5
(7.2, NR)
5.1
(4.4, 6.0)
NR=Not Reached, CI=Confidence Interval
Figure 2. Kaplan-Meier curves of OS in POSEIDON
-
16 HOW SUPPLIED/STORAGE AND HANDLING
IMJUDO (tremelimumab-actl) injection is a clear to slightly opalescent, colorless to slightly yellow solution supplied in a carton containing one single-dose vial in the following concentrations:
- 25 mg/1.25 mL (20 mg/mL) (NDC: 0310-4505-25)
- 300 mg/15 mL (20 mg/mL) (NDC: 0310-4535-30)
Store in a refrigerator at 2°C to 8°C (36°F to 46°F) in original carton to protect from light.
Do not freeze. Do not shake.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Inform patients of the risk of immune-mediated adverse reactions that may require corticosteroid treatment and interruption or discontinuation of IMJUDO in combination with durvalumab, including [see Warnings and Precautions (5.1)]:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for any new or worsening cough, chest pain, or shortness of breath.
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea, blood or mucus in stools, or severe abdominal pain.
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, pain on the right side of abdomen, lethargy, or easy bruising or bleeding.
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypothyroidism, hyperthyroidism, adrenal insufficiency, type 1 diabetes mellitus, or hypophysitis.
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis.
- Dermatological Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of severe dermatological reactions.
- Pancreatitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of pancreatitis.
- Other Immune-Mediated Adverse Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of aseptic meningitis, immune thrombocytopenia, myocarditis, hemolytic anemia, myositis, uveitis, keratitis, and myasthenia gravis.
Infusion-Related Reactions:
- Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity:
- Advise females of reproductive potential that IMJUDO can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
- Advise females of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of IMJUDO [see Use in Specific Populations (8.3)].
Lactation:
- Advise female patients not to breastfeed while taking IMJUDO and for 3 months after the last dose [see Warnings and Precautions (5.3) and Use in Specific Populations (8.2)].
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
Manufactured By:
AstraZeneca AB
Södertälje, Sweden SE-15185
US License No. 2059
IMJUDO® is a registered trademark of AstraZeneca group of companies.
©AstraZeneca 2022
-
MEDICATION GUIDE
MEDICATION GUIDE
IMJUDO® (im-JEW-doh)(tremelimumab-actl)
injection
What is the most important information I should know about IMJUDO?
IMJUDO is a medicine that may treat certain cancers by working with your immune system.
IMJUDO in combination with durvalumab can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended.
Call or see your healthcare provider right away if you develop any new or worsening signs or symptoms, including:
Lung problems.
-
- cough
- shortness of breath
- chest pain
Intestinal problems.
- diarrhea (loose stools) or more frequent bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
Liver problems.
- yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach-area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems.
- headaches that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increase sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems.
- decrease in your amount of urine
- blood in your urine
- swelling of your ankles
- loss of appetite
Skin problems.
- rash
- itching
- skin blistering or peeling
- painful sores or ulcers in mouth or nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Pancreas problems.
- pain in your upper stomach-area (abdomen)
- severe nausea or vomiting
- loss of appetite
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with IMJUDO. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
- chest pain, irregular heartbeats, shortness of breath or swelling of ankles
- confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems
- tingling, numbness or weakness of the arms or legs
- double vision, blurry vision, sensitivity to light, eye pain, changes in eye sight
- persistent or severe muscle pain or weakness, muscle cramps, joint pain, joint stiffness or swelling
- low red blood cells, bruising
Infusion reactions that can sometimes be severe or life-threatening. Signs and symptoms of infusion reactions may include:
- chills or shaking
- itching or rash
- flushing
- shortness of breath or wheezing
- dizziness
- feel like passing out
- fever
- back or neck pain
Getting medical treatment right away may help keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during your treatment with IMJUDO. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with IMJUDO, if you have severe side effects.
What is IMJUDO?
IMJUDO is a prescription medicine used to treat adults with:
- a type of liver cancer called unresectable hepatocellular carcinoma (uHCC). IMJUDO may be used in combination with durvalumab when your uHCC cannot be removed by surgery.
- a type of lung cancer called non-small cell lung cancer (NSCLC). IMJUDO may be used in combination with durvalumab and chemotherapy that contains platinum when your NSCLC:
- o has spread to other part of your body (metastatic), and
- o your tumor does not have an abnormal “EGFR” or “ALK” gene.
It is not known if IMJUDO is safe and effective in children.
Before you receive IMJUDO, tell your healthcare provider about all of your medical conditions, including if you:
- have immune system problems such as Crohn's disease, ulcerative colitis, or lupus
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- are pregnant or plan to become pregnant. IMJUDO can harm your unborn baby.
Females who are able to become pregnant
-
- o Your healthcare provider should do a pregnancy test before you start treatment with IMJUDO.
- o You should use an effective method of birth control during your treatment and for 3 months after your last dose of IMJUDO. Talk to your healthcare provider about birth control methods that you can use during this time.
- o Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with IMJUDO.
- are breastfeeding or plan to breastfeed. It is not known if IMJUDO passes into your breast milk. Do not breastfeed during treatment and for 3 months after your last dose of IMJUDO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive IMJUDO?
- Your healthcare provider will determine your treatment schedule and cycles of treatment.
- Your healthcare provider will give you IMJUDO into your vein through an intravenous (IV) line over 60 minutes.
-
For the treatment of uHCC:
- o On the same day you receive IMJUDO, you will receive durvalumab through an intravenous (IV) line over 60 minutes.
- o IMJUDO is given to you as a single dose.
- o You will then receive durvalumab every 4 weeks
-
For the treatment of NSCLC:
- o On the same day you receive IMJUDO, you will receive durvalumab followed by platinum-containing chemotherapy. You will receive combination chemotherapy every 3 weeks for four cycles (Cycle 1 to 4).
- o You will then receive durvalumab for one cycle (Cycle 5), and then IMJUDO in combination with durvalumab for one cycle only (Cycle 6).
- o You will then receive durvalumab every 4 weeks.
- o Your healthcare provider will decide if you will also receive additional chemotherapy with each cycle.
- Your healthcare provider will test your blood to check you for certain side effects.
- If you miss your appointment, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of IMJUDO?
IMJUDO can cause serious side effects, including:
See “What is the most important information I should know about IMJUDO?”
The most common side effects of IMJUDO when used in combination with durvalumab in adults with uHCC include:
- rash
- diarrhea
- feeling tired
- itchiness
- muscle or bone pain
- stomach area (abdominal) pain
The most common side effects of IMJUDO when used in combination with durvalumab and platinum-containing chemotherapy in adults with metastatic NSCLC include:
- nausea
- feeling tired or weak
- muscle or bone pain
- decreased appetite
- rash
- diarrhea
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of IMJUDO. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of IMJUDO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about IMJUDO, talk with your healthcare provider. You can ask your healthcare provider for information about IMJUDO that is written for health professionals.
What are the ingredients in IMJUDO?
Active ingredient: tremelimumab-actl
Inactive ingredients: edetate disodium, histidine, L-histidine hydrochloride monohydrate, polysorbate 80, trehalose, and Water for Injection, USP.
Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
Manufactured by: AstraZeneca AB, Södertälje, Sweden SE-15185
US License No. 2059
IMJUDO® is a registered trademark of AstraZeneca group of companies.
For more information, call 1-800-236-9933 or go to www.IMJUDO.com
© AstraZeneca 2024
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 01/2024
-
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 0310-4535-30 Rx only
IMJUDO® 300 mg/15 mL
(tremelimumab-actl) (20 mg/mL)
Injection
For Intravenous Infusion After Dilution
Single-dose vial. Discard unused portion.
Attention Pharmacist:
Dispense the accompanying Medication Guide
to each patient.
AstraZeneca
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 0310-4505-25 Rx only
IMJUDO® 25 mg/1.25 mL
(tremelimumab-actl) (20 mg/mL)
Injection
For Intravenous Infusion After Dilution
Single-dose vial. Discard unused portion.
Attention Pharmacist:
Dispense the accompanying Medication Guide
to each patient.
AstraZeneca
-
INGREDIENTS AND APPEARANCE
IMJUDO
tremelimumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-4505 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREMELIMUMAB (UNII: QEN1X95CIX) (TREMELIMUMAB - UNII:QEN1X95CIX) TREMELIMUMAB 25 mg in 1.25 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.1 mg in 1.25 mL HISTIDINE (UNII: 4QD397987E) 1 mg in 1.25 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 4 mg in 1.25 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1.25 mL TREHALOSE (UNII: B8WCK70T7I) 105 mg in 1.25 mL WATER (UNII: 059QF0KO0R) 1.2 g in 1.25 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-4505-25 1 in 1 CARTON 10/21/2022 1 1.25 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761289 10/21/2022 IMJUDO
tremelimumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-4535 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREMELIMUMAB (UNII: QEN1X95CIX) (TREMELIMUMAB - UNII:QEN1X95CIX) TREMELIMUMAB 300 mg in 15 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 1.5 mg in 15 mL HISTIDINE (UNII: 4QD397987E) 10 mg in 15 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 49 mg in 15 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 3 mg in 15 mL TREHALOSE (UNII: B8WCK70T7I) 1260 mg in 15 mL WATER (UNII: 059QF0KO0R) 14 g in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-4535-30 1 in 1 CARTON 10/21/2022 1 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761289 10/21/2022 Labeler - AstraZeneca Pharmaceuticals LP (054743190)
Trademark Results [IMJUDO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMJUDO 90769621 not registered Live/Pending |
AstraZeneca AB 2021-06-11 |
 IMJUDO 86484935 5131050 Live/Registered |
AstraZeneca AB 2014-12-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.