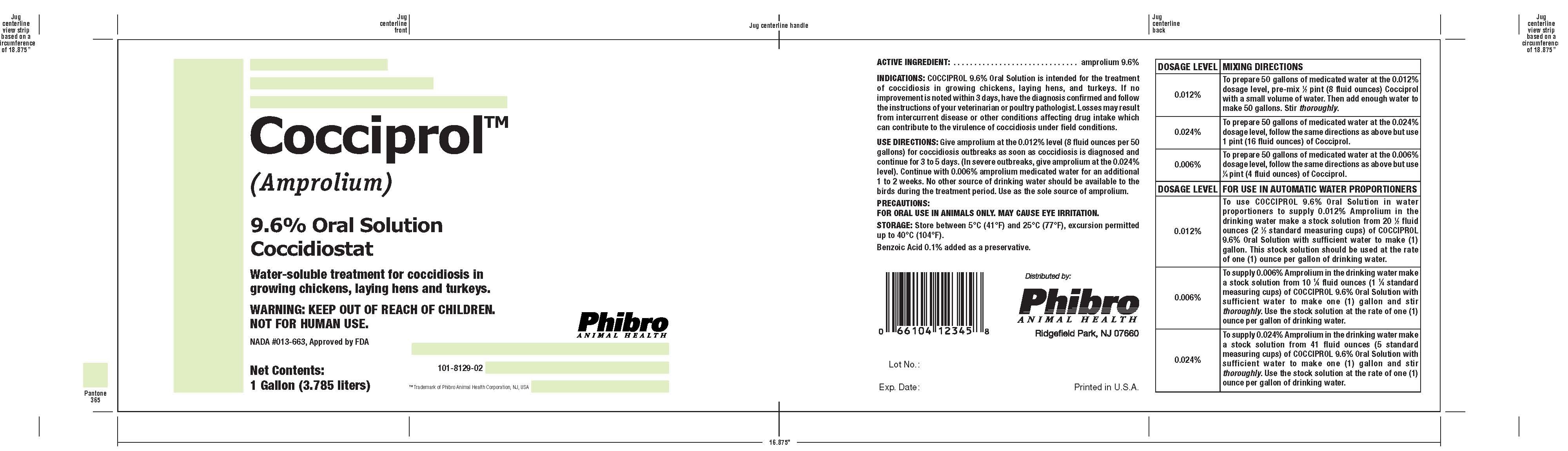
Cocciprol®(Amprolium)
Cocciprol by
Drug Labeling and Warnings
Cocciprol by is a Animal medication manufactured, distributed, or labeled by Phibro Animal Health. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COCCIPROL- amprolium solution
Phibro Animal Health
----------
Cocciprol®(Amprolium)
9.6% Oral Solution
Coccidiostat
Water-soluble treatment for coccidiosis ingrowing chickens, laying hens and turkeys.
INDICATIONS:
COCCIPROL 9.6% Oral Solution is intended for the treatment of coccidiosis in growing chickens, laying hens, and turkeys. lf no improvement is noted within 3 days, have the diagnosis confirmed and follow the instructions of your veterinarian or poultry pathologist. Losses may result from intercurrent disease or other conditions affecting drug intake which can contribute to the virulence of coccidiosis under field conditions.
USE DIRECTIONS:
Give amprolium at the 0.012% level (8 fluid ounces per 50 gallons) for coccidiosis outbreaks as soon as coccidiosis is diagnosed and continue for 3 to 5 days. (In severe outbreaks, give amprolium at the 0.024% level). Continue with 0.006% amprolium medicated water for an additional 1 to 2 weeks. No other source of drinking water should be available to the birds during the treatment period. Use as the sole source of amprolium.
|
DOSAGE LEVEL |
MIXING DIRECTIONS |
|
0.012% |
To prepare 50 gallons of medicated water at the 0.012% dosage level, pre-mix 1⁄2 pint (8 fluid ounces) Cocciprol with a small volume of water. Then add enough water to make 50 gallons. Stir thoroughly. |
|
0.024% |
To prepare 50 gallons of medicated water at the 0.024% dosage level, follow the same directions as above but use 1 pint (16 fluid ounces) of CocciproI. |
|
0.006% |
To prepare 50 gallons of medicated water at the 0.006% dosage level, follow the same directions as above but use 1⁄4 pint (4 fluid ounces) of CocciproI. |
|
DOSAGE LEVEL |
FOR USE IN AUTOMATIC WATER PROPORTIONERS |
|
0.012% |
To use COCCIPROL 9.6% Oral Solution in water proportioners to supply 0.012% Amprolium in the drinking water make a stock solution from 20 1⁄2 fluid ounces (2 1⁄2 standard measuring cups) of COCCIPROL 9.6% Oral Solution with sufficient water to make (1) gallon. This stock solution should be used at the rate of one (1) ounce per gallon of drinking water. |
|
0.006% |
To supply 0.006% Amprolium in the drinking water make a stock solution from 10 1⁄4 fluid ounces (1 1⁄4 standard measuring cups) of COCCIPROL 9.6% Oral Solution with sufficient water to make one (1) gallon and stir thoroughly. Use the stock solution at the rate of one (1) ounce per gallon of drinking water. |
|
0.024% |
To supply 0.024% Amprolium in the drinking water make a stock solution from 41 fluid ounces (5 standard measuring cups) of COCCIPROL 9.6% Oral Solution with sufficient water to make one (1) gallon and stir thoroughly. Use the stock solution at the rate of one (1) ounce per gallon of drinking water. |
NADA #013-663, Approved by FDA
Net Contents:
1 Gallon (3.785 liters)
101-8129-02
® Trademark of Phibro Animal Health, NJ, USA
| COCCIPROL
amprolium solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Phibro Animal Health (006989008) |
| Registrant - Phibro Animal Health (006989008) |