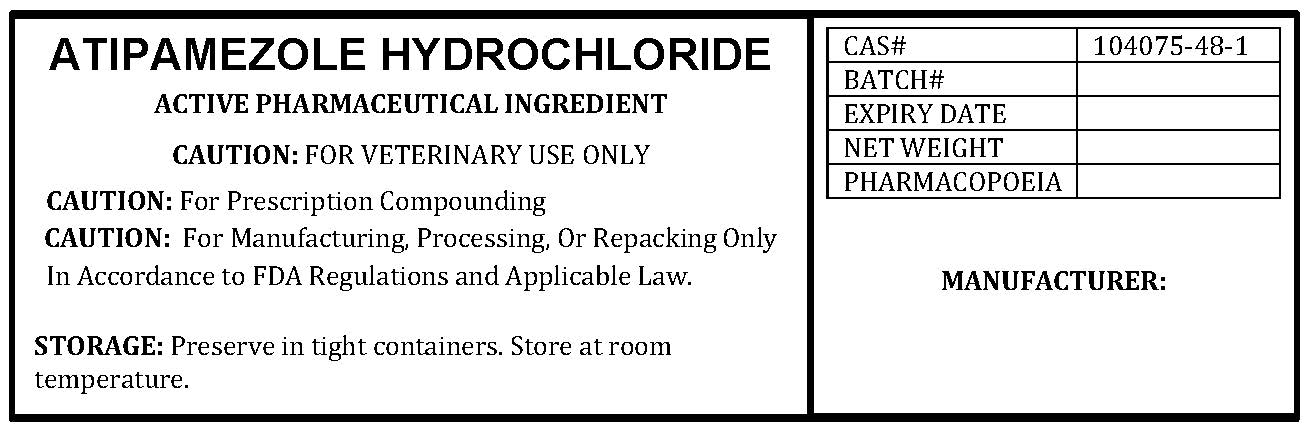
ATIPAMEZOLE HYDROCHLORIDE
ATIPAMEZOLE HYDROCHLORIDE by
Drug Labeling and Warnings
ATIPAMEZOLE HYDROCHLORIDE by is a Other medication manufactured, distributed, or labeled by MAEDA INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ATIPAMEZOLE HYDROCHLORIDE- atipamezole hydrochloride powder
MAEDA INC
----------
ATIPAMEZOLE HYDROCHLORIDE
| ATIPAMEZOLE HYDROCHLORIDE
atipamezole hydrochloride powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - MAEDA INC (118984873) |
Revised: 10/2023
Document Id: 0eaa3d19-ba2a-4c0d-90e9-c2d2fe027dee
Set id: 66d435d1-8643-42bb-98a7-4f214acbec75
Version: 2
Effective Time: 20231023
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.